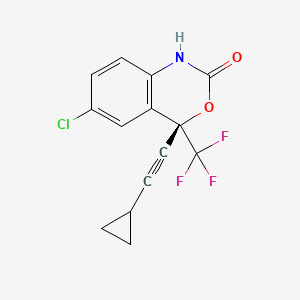
Efavirenz
Overview
Description
Efavirenz is a non-nucleoside reverse transcriptase inhibitor used primarily in the treatment and prevention of human immunodeficiency virus (HIV) type 1 infection . It is marketed under brand names such as Sustiva and Stocrin . This compound works by inhibiting the activity of reverse transcriptase, an enzyme crucial for the replication of HIV .
Mechanism of Action
Target of Action
Efavirenz is a non-nucleoside reverse transcriptase inhibitor (NNRTI) used to treat human immunodeficiency virus (HIV) type 1 infection . The primary target of this compound is the reverse transcriptase enzyme of HIV-1 . This enzyme plays a crucial role in the life cycle of the virus, as it is responsible for transcribing the viral RNA into DNA, a necessary step for the virus to replicate within host cells .
Mode of Action
This compound works by binding to the reverse transcriptase enzyme, thereby blocking its function . This non-competitive inhibition of the enzyme prevents the transcription of viral RNA into DNA, effectively halting the replication of the virus . As a result, the amount of HIV in the blood decreases .
Biochemical Pathways
This compound primarily affects the reverse transcription pathway of the HIV life cycle . By inhibiting the reverse transcriptase enzyme, this compound prevents the conversion of viral RNA into DNA, a critical step in the replication of the virus . This disruption in the viral life cycle leads to a decrease in viral load and slows the progression of the disease .
Pharmacokinetics
This compound exhibits a bioavailability of 40-45% under fasting conditions . It is highly protein-bound (99.5-99.75%) and is metabolized in the liver, primarily by the CYP2A6 and CYP2B6 enzymes . The onset of action occurs within 3-5 hours, and the drug has an elimination half-life of 40-55 hours . This compound is excreted via the kidneys (14-34%) and feces (16-61%) .
Result of Action
The molecular and cellular effects of this compound’s action include a decrease in the amount of HIV in the blood, leading to a reduction in viral load . In the context of cancer, this compound has been shown to cause cell death, retard cell proliferation, and change cell morphology to an epithelial-like phenotype in a range of triple-negative breast cancer (TNBC) cell lines .
Action Environment
Environmental factors can influence the action, efficacy, and stability of this compound. For instance, the presence of other drugs can lead to interactions that affect the metabolism and effectiveness of this compound . Additionally, genetic variations in the enzymes that metabolize this compound, such as CYP2B6, can lead to differences in drug response among individuals .
Biochemical Analysis
Biochemical Properties
Efavirenz interacts with several enzymes and proteins. It is primarily metabolized by cytochrome P450 enzymes, including CYP2B6, CYP2A6, and CYP3A4 . This compound acts as a non-competitive inhibitor of HIV-1 reverse transcriptase . It binds to this enzyme, blocking its function and preventing the replication of HIV .
Cellular Effects
This compound has various effects on cells. It has been associated with neurological and neuropsychiatric reactions ranging from transitory effects such as nightmares, dizziness, insomnia, nervousness, and lack of concentration, to more severe symptoms including depression, suicidal ideation, or even psychosis . This compound has also been linked to mild/moderate neurocognitive impairment .
Molecular Mechanism
This compound exerts its effects at the molecular level by blocking the function of reverse transcriptase, an enzyme crucial for the replication of HIV . It binds non-competitively to the hydrophobic region of the HIV-1 reverse transcriptase through an allosteric mechanism that alters the enzyme conformation and prevents access to the substrates .
Temporal Effects in Laboratory Settings
The majority of this compound-induced CNS effects appear early, even after a single dose, and mitigate within 1 month . In a substantial number of cases, effects such as abnormal dreams, anxiety, or depression persist for much longer periods and require a change of treatment .
Dosage Effects in Animal Models
In animal models, perinatal this compound exposure resulted in reduced body weight and delayed reflex and motor development . During adulthood, a decrease in the total number of cells and mature neurons in the motor cortex was observed, as well as an increase in the number of Caspase-3-positive cells and serotonergic fibers .
Metabolic Pathways
This compound is involved in several metabolic pathways. It is primarily metabolized by CYP2B6 into 8-hydroxythis compound and to a lesser extent via pathways involving CYP2A6, CYP3A4/5, and UGT2B7 .
Preparation Methods
Synthetic Routes and Reaction Conditions: One of the key synthetic routes for efavirenz involves the ortho-lithiation reaction of N-Boc-4-chloroaniline using n-butyllithium . This reaction is performed at a temperature of -45°C and yields a key intermediate, which is then subjected to trifluoroacetylation . Another method involves the reaction of an this compound intermediate with phosgene in solvents such as heptane, toluene, or tetrahydrofuran .
Industrial Production Methods: Industrial production of this compound often employs continuous flow chemistry to improve the selectivity and safety of the reactions . This method allows for the efficient scale-up of the process, making it suitable for large-scale production.
Chemical Reactions Analysis
Types of Reactions: Efavirenz undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions to form different metabolites.
Reduction: Reduction reactions can modify the functional groups present in this compound.
Substitution: Substitution reactions can occur at the chloro or trifluoromethyl groups in the molecule.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Reduction: Reducing agents such as lithium aluminum hydride can be used.
Substitution: Reagents like sodium hydroxide or other strong bases can facilitate substitution reactions.
Major Products Formed: The major products formed from these reactions depend on the specific conditions and reagents used. For example, oxidation can lead to the formation of hydroxylated metabolites, while reduction can yield dechlorinated or defluorinated products.
Scientific Research Applications
Efavirenz has a wide range of scientific research applications:
Chemistry: It is used as a model compound in studies involving non-nucleoside reverse transcriptase inhibitors.
Biology: this compound is studied for its effects on cellular processes and its interactions with various biological molecules.
Medicine: It is a key component of highly active antiretroviral therapy (HAART) for HIV treatment.
Industry: this compound is used in the pharmaceutical industry for the development of antiretroviral drugs and formulations.
Comparison with Similar Compounds
Nevirapine: Another non-nucleoside reverse transcriptase inhibitor used in HIV treatment.
Delavirdine: Similar to efavirenz, it inhibits reverse transcriptase but has different pharmacokinetic properties.
Etravirine: A newer non-nucleoside reverse transcriptase inhibitor with a broader resistance profile.
Uniqueness of this compound: this compound is unique due to its high potency and long half-life, which allows for once-daily dosing . It has been a primary first-line antiviral drug for over 15 years and is known for its effectiveness in combination therapy . its use is sometimes limited by side effects such as central nervous system symptoms .
Properties
IUPAC Name |
(4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1H-3,1-benzoxazin-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XPOQHMRABVBWPR-ZDUSSCGKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC1C#CC2(C3=C(C=CC(=C3)Cl)NC(=O)O2)C(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1CC1C#C[C@]2(C3=C(C=CC(=C3)Cl)NC(=O)O2)C(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H9ClF3NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID9046029 | |
| Record name | Efavirenz | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9046029 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
315.67 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Efavirenz | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014763 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Practically insoluble in water (less than 10 mg/L), 8.55e-03 g/L | |
| Record name | EFAVIRENZ | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7163 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Efavirenz | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014763 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Similar to zidovudine, efavirenz inhibits the activity of viral RNA-directed DNA polymerase (i.e., reverse transcriptase). Antiviral activity of efavirenz is dependent on intracellular conversion to the active triphosphorylated form. The rate of efavirenz phosphorylation varies, depending on cell type. It is believed that inhibition of reverse transcriptase interferes with the generation of DNA copies of viral RNA, which, in turn, are necessary for synthesis of new virions. Intracellular enzymes subsequently eliminate the HIV particle that previously had been uncoated, and left unprotected, during entry into the host cell. Thus, reverse transcriptase inhibitors are virustatic and do not eliminate HIV from the body. Even though human DNA polymerase is less susceptible to the pharmacologic effects of triphosphorylated efavirenz, this action may nevertheless account for some of the drug's toxicity., Efavirenz diffuses into the cell where it binds adjacent to the active site of reverse transcriptase. This produces a conformational change in the enzyme that inhibits function. | |
| Record name | Efavirenz | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00625 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | EFAVIRENZ | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7163 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from toluene:heptane, White to slightly pink crystalline powder | |
CAS No. |
154598-52-4 | |
| Record name | Efavirenz | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=154598-52-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Efavirenz [USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0154598524 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Efavirenz | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00625 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | 154598-52-4 | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=742403 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Efavirenz | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9046029 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2,4-dihydro-1H-3,1-benzoxazin-2-one | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | EFAVIRENZ | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/JE6H2O27P8 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | EFAVIRENZ | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7163 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Efavirenz | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014763 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
139-141 °C, 139 - 141 °C | |
| Record name | Efavirenz | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00625 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | EFAVIRENZ | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7163 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Efavirenz | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014763 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: Efavirenz is a non-nucleoside reverse transcriptase inhibitor (NNRTI) that binds non-competitively to the reverse transcriptase enzyme of HIV-1. [, , , ] This binding occurs in a hydrophobic pocket within the p66 subunit of the enzyme, distinct from the binding site of nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs). [] this compound's binding alters the conformation of critical amino acids in the enzyme's catalytic site, inhibiting the conversion of viral RNA into DNA and, therefore, viral replication. []
A: By inhibiting reverse transcriptase, this compound prevents the formation of proviral DNA, effectively halting the replication cycle of HIV-1. [] This inhibition leads to a decrease in viral load and an increase in CD4+ T-cell count in infected individuals. [, , , ]
ANone: this compound has the molecular formula C14H9ClF3NO2 and a molecular weight of 315.68 g/mol.
A: Yes, physiologically based pharmacokinetic (PBPK) simulations have been employed to model this compound distribution in various body compartments, including plasma, cerebrospinal fluid, and brain tissue. [] These models can be valuable tools for predicting drug exposure and potentially optimizing dosing regimens.
A: While the provided abstracts don't delve into specific SAR studies, they highlight that modifications to the this compound molecule can impact its pharmacological properties. For instance, this compound is metabolized by CYP2B6 and CYP3A4 enzymes. [, ] Genetic polymorphisms in these enzymes, particularly CYP2B6, significantly influence this compound plasma concentrations, potentially impacting efficacy and toxicity. [, , , , , ] This emphasizes the importance of SAR studies in understanding and optimizing the therapeutic profile of this compound.
A: One abstract explores the development of a solid drug nanoformulation (SDN) of this compound. [] This nanoformulation aimed to mitigate this compound-associated limitations, potentially improving its bioavailability and reducing toxicity.
A: this compound is administered orally and is well-absorbed. [, , , ] It undergoes extensive metabolism primarily by CYP3A4 and CYP2B6 enzymes in the liver, with 8-hydroxythis compound as the major metabolite. [, , , , ]
ANone: Numerous factors can impact this compound pharmacokinetics, including:
- Genetic factors: Polymorphisms in CYP2B6 are particularly influential on this compound plasma levels. [, , , , , , ] Individuals with certain CYP2B6 genotypes may exhibit significantly higher or lower this compound exposure.
- Concomitant medications: Drugs that induce or inhibit CYP3A4 and CYP2B6 can alter this compound metabolism, leading to interactions. [, , , , , , ]
- Ethnicity: Studies show variations in this compound pharmacokinetics across different ethnic groups. [, , ] For example, some studies observed higher this compound plasma concentrations in Black African patients compared with other populations.
- Body weight: Obesity, particularly abdominal obesity, has been associated with lower this compound plasma concentrations. []
- Liver function: Liver cirrhosis may lead to elevated this compound plasma levels due to impaired metabolism. [, ]
A: Yes, in vitro models, including primary rat neurons and Hep3B cells (a human liver cell line), have been used to investigate the potential toxicity of this compound and compare its profile to newer antiretrovirals. []
A: The most frequent HIV-1 mutation associated with this compound treatment failure is the K103N substitution in the reverse transcriptase gene. [, ] This mutation, often emerging alongside other NNRTI resistance mutations, confers cross-resistance to nevirapine, another NNRTI. [, , ]
A: Studies suggest that the use of thymidine analogue NRTIs (like zidovudine or stavudine) alongside nevirapine might be linked to a higher selection of mutations conferring cross-resistance to this compound. []
A: The most prominent concern with this compound is its potential for neuropsychiatric adverse events, including: [, , , ] * Abnormal dreams * Sleep disturbances * Nervousness * Anxiety * Depression * Dizziness
A: Research suggests that:* This compound plasma concentrations: Monitoring this compound plasma levels can be valuable for guiding dose adjustments, particularly in individuals at risk of toxicity or subtherapeutic exposure. [, , , ] * Genetic polymorphisms: CYP2B6 genotypes are considered strong predictors of this compound plasma concentrations and may be used to personalize therapy. [, , , , ]* Baseline liver function tests: Pre-existing liver disease, particularly cirrhosis, is associated with altered this compound pharmacokinetics and may require closer monitoring for potential toxicity. []
ANone: Several analytical methods are utilized in this compound studies:
- High-performance liquid chromatography (HPLC): This technique is widely used to measure this compound concentrations in biological matrices, such as plasma. [, , , , , ]
- Liquid chromatography-mass spectrometry (LC-MS): This highly sensitive and specific method allows for the simultaneous quantification of this compound and other antiretroviral drugs in complex biological samples. [, , ]
- Allele-specific PCR (AS-PCR): This molecular technique is used to detect specific HIV-1 mutations, such as the K103N mutation associated with this compound resistance. []
A: While not directly addressed in the provided research, one abstract suggests that this compound does not meet the criteria for a rapidly dissolving product according to the Biopharmaceutics Classification System. [] Factors such as excipients and manufacturing processes can affect this compound absorption, underscoring the importance of dissolution studies for ensuring consistent bioavailability.
A: Evidence suggests that this compound might interact with drug transporters. One study observed a significant reduction in cellular this compound accumulation in the presence of transporter inhibitors like montelukast (an inhibitor of organic anion transporting polypeptide 1B1) and amantadine (an inhibitor of organic cation transporter 1). [] This suggests that this compound could be a substrate for these transporters.
ANone: this compound exhibits a complex interplay of inhibitory and inducing effects on drug-metabolizing enzymes:
- Inhibition: In vitro studies demonstrate that this compound inhibits various CYP450 enzymes, including CYP2C9, CYP2C19, CYP3A4, CYP2D6, and CYP1A2. []
A: Yes, several alternative NNRTIs exist, including: * Nevirapine: While also effective, nevirapine has its own set of potential side effects and drug interactions. [, , , , ] * Rilpivirine: This newer NNRTI shows a potentially safer toxicological profile than this compound. [] * Etravirine: In clinical trials, etravirine demonstrated comparable efficacy to this compound with a potentially more favorable neuropsychiatric profile. []
ANone: Yes, several research areas highlight the cross-disciplinary nature of this compound research:
- Pharmacogenetics: This field combines pharmacology and genetics to understand the impact of genetic variations on drug response, as seen with the significant influence of CYP2B6 polymorphisms on this compound pharmacokinetics. [, , , , , ]
- Drug formulation: Nanoformulations of this compound, as mentioned in one abstract, demonstrate the intersection of pharmaceutical sciences and nanotechnology to improve drug delivery and therapeutic outcomes. []
- Cellular and molecular biology: In vitro studies using cell lines and primary cells are essential for investigating the mechanisms of this compound action and toxicity, bridging pharmacology with cellular and molecular biology. []
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


![1-(Carbamoylamino)-1-oxopropan-2-yl 3-[(3,4-dimethoxyphenyl)methylidene]-1H,2H,3H-cyclopenta[b]quinoline-9-carboxylate](/img/structure/B1671038.png)
![(10S,13R,14R,17R)-4,4,10,13,14-pentamethyl-17-[(2R)-6-methyl-5-methylideneheptan-2-yl]-1,2,5,6,7,11,12,15,16,17-decahydrocyclopenta[a]phenanthren-3-one](/img/structure/B1671042.png)