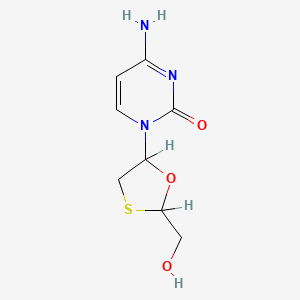
Lamivudine
Overview
Description
Lamivudine, also known as 2’,3’-dideoxy-3’-thiacytidine, is a synthetic nucleoside analogue with potent antiviral properties. It is primarily used to treat infections caused by the Human Immunodeficiency Virus (HIV) and the Hepatitis B Virus (HBV). This compound is a nucleoside reverse transcriptase inhibitor that works by blocking the reverse transcriptase enzyme, which is essential for viral replication .
Mechanism of Action
Target of Action
Lamivudine, also known as 3TC, is a synthetic nucleoside analogue . It primarily targets two key enzymes: HIV reverse transcriptase and hepatitis B virus polymerase . These enzymes are crucial for the replication of HIV and hepatitis B virus (HBV), respectively .
Mode of Action
This compound is phosphorylated intracellularly to its active 5’-triphosphate metabolite, this compound triphosphate (L-TP) . This nucleoside analogue is incorporated into viral DNA by HIV reverse transcriptase and HBV polymerase . The incorporation of L-TP into the growing viral DNA chain results in DNA chain termination , thereby inhibiting viral replication .
Biochemical Pathways
This compound’s principal mode of action involves the inhibition of the reverse transcription process of the HIV-1 and HBV life cycles . Reverse transcription is the process by which the viral RNA genome is converted into DNA. This compound, in its active triphosphate form, competes with the natural substrate, cytosine triphosphate, for incorporation into the growing viral DNA chain . Once incorporated, it causes premature termination of the DNA chain, thus preventing the completion of viral DNA synthesis .
Pharmacokinetics
This compound is well absorbed from the gastrointestinal tract, and its bioavailability is approximately 86% . It is less than 36% bound to plasma proteins . The elimination half-life of this compound is between 5 to 7 hours, and about 70% of the drug is excreted unchanged in urine . These properties contribute to the drug’s good bioavailability and allow for effective therapeutic concentrations to be achieved in the body .
Result of Action
The primary molecular effect of this compound is the inhibition of viral DNA synthesis, which leads to a decrease in viral load and an increase in CD4 cell counts in HIV-infected patients . At the cellular level, this compound has been shown to inhibit cell growth and reduce the invasive capability of certain cancer cells . Additionally, it has been found to downregulate the expression of human endogenous retroviruses (HERVs), which may have implications in various diseases .
Action Environment
The action of this compound can be influenced by various environmental factors. For instance, the presence of renal impairment can affect the drug’s excretion, necessitating dose adjustment . Furthermore, the efficacy of this compound can be affected by the presence of viral resistance mutations . This compound is typically used in combination with other antiretroviral drugs to enhance efficacy and prevent the development of drug resistance . It can be taken with or without food , and should be stored at room temperature .
Biochemical Analysis
Biochemical Properties
Lamivudine interacts with various enzymes and proteins within the cell. It is phosphorylated intracellularly to its active 5’-triphosphate metabolite, this compound triphosphate (L-TP) . This metabolite is incorporated into viral DNA by HIV reverse transcriptase and HBV polymerase, resulting in DNA chain termination .
Cellular Effects
This compound has significant effects on various types of cells and cellular processes. It influences cell function by inhibiting the replication of HIV-1 and HBV, thereby reducing the viral load within the cell . This can impact cell signaling pathways, gene expression, and cellular metabolism.
Molecular Mechanism
This compound exerts its effects at the molecular level through several mechanisms. It is incorporated into viral DNA by HIV reverse transcriptase and HBV polymerase, resulting in DNA chain termination . This prevents the replication of the virus and reduces the viral load within the cell.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound have been observed to change over time. This compound shows a burst release at the beginning and sustained release until 24 hours in physiological conditions . Low pH can accelerate the release of this compound .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. For instance, this compound has been shown to improve cognition in a mouse model of Down syndrome
Metabolic Pathways
This compound is involved in several metabolic pathways. It undergoes anabolic phosphorylation by intracellular kinases to form its active 5’-triphosphate metabolite, this compound triphosphate (L-TP) . This metabolite is then incorporated into viral DNA, impacting metabolic flux and metabolite levels within the cell.
Transport and Distribution
This compound is transported and distributed within cells and tissues. It is rapidly absorbed after oral administration, with maximum serum concentrations usually attained 0.5 to 1.5 hours after the dose . It is widely distributed into total body fluid .
Preparation Methods
Synthetic Routes and Reaction Conditions: Lamivudine can be synthesized through various methods, including enantioselective synthesis and dynamic kinetic resolution. One common method involves the use of L-menthyl glyoxylate as a chiral auxiliary. The synthesis typically includes esterification of menthol with carboxyl derivatives such as glyoxyl acid, followed by ozonolysis and hydration .
Industrial Production Methods: In industrial settings, this compound is produced using large-scale chemical synthesis processes that ensure high yield and purity. The process involves multiple steps, including the preparation of intermediates, purification, and crystallization to obtain the final product .
Chemical Reactions Analysis
Types of Reactions: Lamivudine undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized to form its corresponding sulfoxide and sulfone derivatives.
Reduction: Reduction reactions can convert this compound into its dihydro derivatives.
Substitution: Nucleophilic substitution reactions can modify the thiol group in this compound to form different analogues.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are used.
Substitution: Nucleophiles like alkyl halides and thiolates are employed in substitution reactions.
Major Products: The major products formed from these reactions include sulfoxides, sulfones, dihydro derivatives, and various substituted analogues .
Scientific Research Applications
Lamivudine has a wide range of scientific research applications:
Chemistry: It is used as a model compound in studies of nucleoside analogues and their chemical properties.
Biology: this compound is employed in research on viral replication mechanisms and the development of antiviral therapies.
Medicine: It is a key component in the treatment of HIV and HBV infections, often used in combination with other antiretroviral drugs.
Industry: this compound is used in the pharmaceutical industry for the development of antiviral medications and formulations
Comparison with Similar Compounds
Emtricitabine: Another nucleoside reverse transcriptase inhibitor with a similar mechanism of action.
Zidovudine: An older nucleoside analogue used in the treatment of HIV.
Abacavir: A nucleoside reverse transcriptase inhibitor often used in combination with lamivudine
Uniqueness: this compound is unique due to its high efficacy against both HIV-1 and HIV-2, as well as its ability to treat chronic hepatitis B. Its favorable safety profile and low incidence of side effects make it a preferred choice in many antiretroviral therapy regimens .
Properties
IUPAC Name |
4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C8H11N3O3S/c9-5-1-2-11(8(13)10-5)6-4-15-7(3-12)14-6/h1-2,6-7,12H,3-4H2,(H2,9,10,13)/t6-,7+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JTEGQNOMFQHVDC-NKWVEPMBSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1C(OC(S1)CO)N2C=CC(=NC2=O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1[C@H](O[C@H](S1)CO)N2C=CC(=NC2=O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C8H11N3O3S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7023194 | |
| Record name | Lamivudine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023194 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
229.26 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Lamivudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014847 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
In water, 70,000 mg/L @ 20 °C, 2.76e+00 g/L | |
| Record name | Lamivudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00709 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LAMIVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7155 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Lamivudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014847 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
8.3X10-16 mm Hg @ 25 °C /Estimated/ | |
| Record name | LAMIVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7155 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Lamivudine is a synthetic nucleoside analogue and is phosphorylated intracellularly to its active 5'-triphosphate metabolite, lamivudine triphosphate (L-TP). This nucleoside analogue is incorporated into viral DNA by HIV reverse transcriptase and HBV polymerase, resulting in DNA chain termination., Lamivudine enters cells by passive diffusion and is phosphorylated to its active metabolite, lamivudine triphosphate. Lamivudine triphosphate competes with deoxycytidine triphosphate for binding to reverse transcriptase, and incorporation into DNA results in chain termination. Lamivudine has very low affinity for human alpha and omega DNA polymerases, moderate affinity for beta DNA polymerase, and higher affinity for gamma DNA polymerase. | |
| Record name | Lamivudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00709 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LAMIVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7155 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from boiling ethanol | |
CAS No. |
134678-17-4 | |
| Record name | Lamivudine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=134678-17-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Lamivudine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0134678174 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Lamivudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00709 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Lamivudine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023194 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | LAMIVUDINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/2T8Q726O95 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | LAMIVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7155 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Lamivudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014847 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
160-162 °C, 160 - 162 °C | |
| Record name | Lamivudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00709 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LAMIVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7155 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Lamivudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014847 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does lamivudine inhibit HBV replication?
A1: this compound is a cytidine analogue that inhibits HBV replication by acting as a chain terminator of viral DNA synthesis. [] Once inside the cell, this compound is phosphorylated to its active triphosphate form. This active form competes with natural cytidine triphosphate for incorporation into the growing viral DNA chain by HBV's reverse transcriptase. Due to its structural difference from cytidine, this compound lacks the 3'-hydroxyl group necessary for further chain elongation, effectively halting viral DNA synthesis. []
Q2: What are the downstream effects of this compound treatment in patients with CHB?
A2: The primary downstream effect of this compound treatment is the suppression of HBV DNA replication. [] This often leads to a decrease in serum alanine aminotransferase (ALT) levels, indicating reduced liver inflammation. [] In some patients, this compound treatment can also lead to HBeAg seroconversion, a marker of long-term response to treatment and a more favorable disease course. [] Additionally, this compound therapy has been shown to improve liver histology in CHB patients. []
Q3: What is the molecular formula and weight of this compound?
A3: The molecular formula of this compound is C8H11N3O3S, and its molecular weight is 229.26 g/mol. []
Q4: How do mutations in the HBV polymerase gene affect this compound's activity?
A4: Mutations in the reverse transcriptase (RT) domain of the HBV polymerase gene, specifically the YMDD motif, are the primary mechanism of this compound resistance. [] The most common mutation is rtM204V/I, where methionine at position 204 is replaced by valine or isoleucine. This mutation reduces this compound's binding affinity to the RT, hindering its incorporation into viral DNA and leading to reduced drug efficacy. [, ]
Q5: What are the limitations of this compound's long-term use in CHB treatment?
A6: One major limitation is the emergence of drug-resistant HBV mutants, particularly those with mutations in the YMDD motif of the polymerase gene. [] These mutations can lead to virological breakthrough and disease reactivation. [] The rate of this compound resistance can be as high as 70% after four years of treatment. []
Q6: How is this compound absorbed and eliminated from the body?
A7: this compound is well absorbed orally, reaching peak plasma concentrations within 1 hour after administration. [] It is primarily eliminated unchanged in the urine, with approximately 78% of the administered dose recovered renally. []
Q7: What are the typical virologic response rates to this compound in patients with CHB?
A9: Virologic response rates to this compound vary depending on factors like HBV genotype, pretreatment ALT levels, and HBeAg status. [, , ] Studies show that after one year of treatment, approximately 18-30% of HBeAg-positive patients achieve HBeAg seroconversion. [] In HBeAg-negative patients, a significant proportion achieve undetectable HBV DNA levels. []
Q8: Has this compound been studied in combination with other antiviral agents?
A10: Yes, this compound has been studied in combination with other antiviral agents, such as interferon-alpha and adefovir. [, ] While the addition of this compound to interferon-alpha did not show significant improvement over this compound monotherapy, sequential therapy (this compound followed by interferon) demonstrated better outcomes. [, ] The combination of this compound and adefovir is effective in treating this compound-resistant CHB. []
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


![10-(2-Chloro-6-fluorobenzyl)-8-(4-methylpiperazine-1-carbonyl)dibenzo[b,f][1,4]thiazepin-11(10H)-one](/img/structure/B1674374.png)