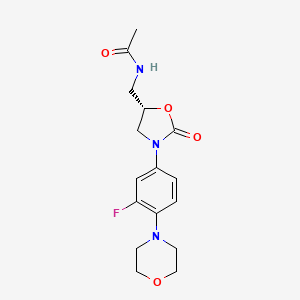
Linezolid
Overview
Description
Linezolid is a synthetic antibiotic belonging to the oxazolidinone class. It is primarily used to treat infections caused by Gram-positive bacteria, including those resistant to other antibiotics such as vancomycin-resistant Enterococcus faecium and methicillin-resistant Staphylococcus aureus . This compound works by inhibiting bacterial protein synthesis, making it a valuable option in the treatment of various bacterial infections .
Mechanism of Action
Target of Action
Linezolid, a synthetic antibiotic, is primarily used to treat infections caused by aerobic Gram-positive bacteria . Its primary targets are the 23S ribosomal RNA of the 50S subunit . These ribosomal subunits play a crucial role in bacterial protein synthesis, making them an effective target for antibiotics like this compound .
Mode of Action
This compound exerts its antibacterial activity by inhibiting the initiation of bacterial protein synthesis . More specifically, it binds to the 23S ribosomal RNA of the 50S subunit . This binding prevents the formation of the 70S initiation complex , which is essential for bacterial reproduction . As a result, this compound is bacteriostatic against both enterococci and staphylococci and bactericidal against most isolates of streptococci .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the bacterial protein synthesis pathway . By inhibiting the formation of the 70S initiation complex, this compound disrupts the protein synthesis process, leading to a halt in bacterial growth and reproduction .
Pharmacokinetics
This compound exhibits excellent pharmacokinetic properties, including 100% bioavailability when administered orally . It is a moderately lipophilic drug and is widely distributed to well-perfused tissues and body fluids . It has a low protein binding capacity of 31% . This compound is primarily metabolized in the liver, with 50-70% of the drug undergoing metabolism . The elimination half-life of this compound is between 3-7 hours . These properties contribute to this compound’s high bioavailability and effectiveness in treating bacterial infections .
Result of Action
The molecular and cellular effects of this compound’s action primarily involve the inhibition of bacterial growth and reproduction. By preventing the formation of the 70S initiation complex, this compound disrupts bacterial protein synthesis, leading to a halt in bacterial growth . This results in the effective treatment of infections caused by susceptible Gram-positive bacteria .
Action Environment
The action of this compound can be influenced by various environmental factors. For instance, the presence of other drugs can impact the pharmacokinetics of this compound . Additionally, the widespread distribution of this compound resistance genes in various niches, including humans, animals, and the environment, can affect the efficacy of this compound . Therefore, understanding these environmental factors is crucial for optimizing the use of this compound in clinical settings .
Biochemical Analysis
Biochemical Properties
Linezolid plays a crucial role in inhibiting bacterial protein synthesis by binding to the 50S ribosomal subunit . This binding prevents the formation of the initiation complex for protein synthesis, thereby halting bacterial growth . This compound interacts with various biomolecules, including ribosomal RNA and proteins involved in the translation process . The nature of these interactions is primarily inhibitory, as this compound disrupts the normal function of the ribosome .
Cellular Effects
This compound exerts significant effects on bacterial cells by inhibiting protein synthesis, leading to cell death . In mammalian cells, this compound can affect mitochondrial protein synthesis due to the similarity between bacterial ribosomes and mitochondrial ribosomes . This can result in mitochondrial dysfunction, impacting cellular metabolism and energy production . This compound has also been shown to influence cell signaling pathways and gene expression related to stress responses and apoptosis .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to the 50S ribosomal subunit, specifically targeting the peptidyl transferase center . This binding inhibits the formation of the initiation complex, preventing the elongation of the nascent peptide chain . This compound’s interaction with ribosomal RNA and proteins is highly specific, leading to the inhibition of protein synthesis . Additionally, this compound can induce changes in gene expression related to stress responses and apoptosis .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound can change over time due to its stability and degradation . This compound is relatively stable, but its long-term effects on cellular function can include mitochondrial dysfunction and altered cellular metabolism . In vitro studies have shown that prolonged exposure to this compound can lead to the accumulation of its metabolites, which may contribute to its long-term effects .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models . At therapeutic doses, this compound effectively inhibits bacterial growth without causing significant toxicity . At higher doses, this compound can cause adverse effects, including hematological toxicity and mitochondrial dysfunction . Threshold effects have been observed, where higher doses lead to more pronounced toxic effects .
Metabolic Pathways
This compound is metabolized primarily in the liver, involving oxidation and reduction reactions . The major metabolites of this compound are excreted in the urine . Enzymes such as cytochrome P450 are involved in the metabolism of this compound, affecting its metabolic flux and metabolite levels . This compound’s interaction with these enzymes can influence its pharmacokinetics and pharmacodynamics .
Transport and Distribution
This compound is transported and distributed within cells and tissues through passive diffusion and active transport mechanisms . It interacts with transporters and binding proteins that facilitate its uptake and distribution . This compound’s localization and accumulation within cells can affect its therapeutic efficacy and toxicity .
Subcellular Localization
This compound’s subcellular localization is primarily within the cytoplasm, where it interacts with ribosomes . It can also localize to mitochondria, affecting mitochondrial protein synthesis and function . Targeting signals and post-translational modifications may direct this compound to specific cellular compartments, influencing its activity and function .
Preparation Methods
Synthetic Routes and Reaction Conditions: Linezolid is synthesized through a multi-step process involving several key intermediates. One common method involves the preparation of ®-N-[[3-[3-fluoro-4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methanol, which is then converted to this compound through a series of reactions . The process typically includes steps such as substitution, cyclization, and amidation .
Industrial Production Methods: Industrial production of this compound involves optimizing the synthetic route to ensure high yield and purity. The process is designed to be cost-effective and environmentally friendly, with minimal waste and by-products . Key steps include the use of metal bases and specific reaction conditions to achieve the desired product .
Chemical Reactions Analysis
Types of Reactions: Linezolid undergoes various chemical reactions, including oxidation, reduction, and substitution . These reactions are essential for its synthesis and modification.
Common Reagents and Conditions: Common reagents used in the synthesis of this compound include potassium phthalimide, metal bases, and specific solvents . Reaction conditions such as temperature, pressure, and pH are carefully controlled to ensure optimal results.
Major Products Formed: The major product formed from these reactions is this compound itself, along with intermediates such as ®-N-[[3-[3-fluoro-4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methanol .
Scientific Research Applications
Linezolid has a wide range of scientific research applications:
Chemistry: In chemistry, this compound is studied for its unique synthetic pathway and its potential as a model compound for developing new antibiotics .
Biology: In biology, this compound is used to study bacterial protein synthesis and resistance mechanisms. It provides insights into how bacteria develop resistance and how new antibiotics can be designed to overcome this challenge .
Medicine: In medicine, this compound is used to treat various bacterial infections, including pneumonia, skin infections, and infections caused by resistant bacteria . It is also studied for its potential use in treating drug-resistant tuberculosis .
Industry: In the pharmaceutical industry, this compound is an important drug for treating serious bacterial infections. Its production and formulation are critical areas of research and development .
Comparison with Similar Compounds
Tedizolid: Another oxazolidinone antibiotic with a similar mechanism of action but different pharmacokinetic properties.
Vancomycin: A glycopeptide antibiotic used to treat Gram-positive infections, but with a different mechanism of action.
Uniqueness of Linezolid: this compound is unique due to its ability to inhibit the initiation of protein synthesis, a mechanism not commonly found in other antibiotics . Its effectiveness against resistant bacteria and its relatively low resistance rates make it a valuable option in the treatment of serious infections .
Properties
IUPAC Name |
N-[[(5S)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H20FN3O4/c1-11(21)18-9-13-10-20(16(22)24-13)12-2-3-15(14(17)8-12)19-4-6-23-7-5-19/h2-3,8,13H,4-7,9-10H2,1H3,(H,18,21)/t13-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
TYZROVQLWOKYKF-ZDUSSCGKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(=O)NCC1CN(C(=O)O1)C2=CC(=C(C=C2)N3CCOCC3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(=O)NC[C@H]1CN(C(=O)O1)C2=CC(=C(C=C2)N3CCOCC3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H20FN3O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5046489 | |
| Record name | Linezolid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5046489 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
337.35 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Linezolid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014739 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
1.44e+00 g/L | |
| Record name | Linezolid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00601 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linezolid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014739 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Linezolid exerts its antibacterial effects by interfering with bacterial protein translation. It binds to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initiation complex, which is essential for bacterial reproduction, thereby preventing bacteria from dividing. Point mutations in the bacterial 23S rRNA can lead to linezolid resistance, and the development of linezolid-resistant _Enterococcus faecium_ and _Staphylococcus aureus_ have been documented during its clinical use. As antimicrobial susceptibility patterns are geographically distinct, local antibiograms should be consulted to ensure adequate coverage of relevant pathogens prior to use., Linezolid is a synthetic oxazolidinone anti-infective agent that is structurally unrelated to other anti-infectives commercially available in the US. In contrast to other anti-infectives that inhibit bacterial protein synthesis, linezolid acts early in translation by binding to a site on the bacterial 23S ribosomal RNA of the 50S subunit and preventing the formation of a functional 70S initiation complex, which is an essential component of the bacterial translation process., Linezolid acts via inhibition of protein synthesis. It bind to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initiation complex. This step is essential for the bacterial translation process., Linezolid is an oxazolidinone antibiotic that is increasingly used to treat drug-resistant, gram-positive pathogens. The mechanism of action is inhibition of bacterial protein synthesis. Optic and/or peripheral neuropathy and lactic acidosis are reported side effects, but the underlying pathophysiological mechanism has not been unravelled. Mitochondrial ultrastructure, mitochondrial respiratory chain enzyme activity /were studied/, and mitochondrial DNA (mtDNA) in muscle, liver, and kidney samples obtained from a patient who developed optic neuropathy, encephalopathy, skeletal myopathy, lactic acidosis, and renal failure after prolonged use of linezolid. In addition, mtDNA, respiratory chain enzyme activity, and protein amount in muscle and liver samples obtained from experimental animals that received linezolid or placebo /were evaluated/. In the patient, mitochondrial respiratory chain enzyme activity was decreased in affected tissues, without ultrastructural mitochondrial abnormalities and without mutations or depletion of mtDNA. In the experimental animals, linezolid induced a dose- and time-dependent decrease of the activity of respiratory chain complexes containing mtDNA-encoded subunits and a decreased amount of protein of these complexes, whereas the amount of mtDNA was normal. These results provide direct evidence that linezolid inhibits mitochondrial protein synthesis with potentially severe clinical consequences. | |
| Record name | Linezolid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00601 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LINEZOLID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7478 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White crystals from ethyl acetate and hexanes | |
CAS No. |
165800-03-3 | |
| Record name | Linezolid | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=165800-03-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Linezolid [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0165800033 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Linezolid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00601 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linezolid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5046489 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | LINEZOLID | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/ISQ9I6J12J | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | LINEZOLID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7478 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Linezolid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014739 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
181.5-182.5 °C | |
| Record name | Linezolid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00601 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LINEZOLID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7478 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Synthesis routes and methods
Procedure details






Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the mechanism of action of Linezolid?
A1: this compound exerts its antibacterial effect by binding to the bacterial ribosome, specifically at the peptidyl transferase center (PTC) located within the 50S ribosomal subunit []. This binding interferes with the formation of the 70S initiation complex, which is essential for protein synthesis [, ].
Q2: What is the downstream effect of this compound binding to the ribosome?
A2: By preventing the formation of the 70S initiation complex, this compound effectively inhibits the initiation phase of bacterial protein synthesis [, ]. This bacteriostatic action prevents bacterial growth and allows the host's immune system to clear the infection.
Q3: What is the molecular formula and weight of this compound?
A3: The molecular formula of this compound is C16H20FN3O4, and its molecular weight is 337.34 g/mol.
Q4: Is there any spectroscopic data available for this compound?
A4: Yes, UV-Spectrophotometric methods have been developed and validated for the quantification of this compound in bulk material and tablet formulations []. These methods utilize the absorbance of this compound in the UV-visible range, with a maximum absorbance observed at 251 nm [].
Q5: Is this compound compatible with commonly used intravenous fluids?
A5: Yes, studies have demonstrated that this compound is stable in commonly used intravenous fluids like sodium lactate, 0.9% sodium chloride, and 5% and 10% glucose solutions for up to 34 days at 25°C [].
Q6: How is this compound eliminated from the body?
A6: Approximately 50% of this compound is eliminated through renal clearance, indicating the importance of renal function in its pharmacokinetics []. The remaining portion is likely metabolized, with further research needed to fully elucidate the metabolic pathways.
Q7: What is the relationship between this compound exposure and thrombocytopenia?
A7: Studies suggest that thrombocytopenia, a decrease in platelet count, is primarily driven by this compound's inhibition of platelet formation (myelosuppression) rather than enhanced platelet destruction []. Higher this compound concentrations (Cmin > 2 mg/L and AUC0-24 > 160 mg*h/L) have been associated with an increased risk of cytopenias, including thrombocytopenia, in patients treated for multidrug-resistant tuberculosis [].
Q8: Does impaired renal function influence this compound pharmacokinetics and the risk of thrombocytopenia?
A8: Yes, impaired renal function can lead to the accumulation of both this compound and its metabolite PNU142300, resulting in higher drug exposure [, ]. This accumulation increases the risk of thrombocytopenia in patients with renal insufficiency [, , ]. Dose adjustments might be necessary for these patients to minimize the risk of adverse effects.
Q9: Does co-administration of rifampin impact this compound exposure?
A9: Yes, rifampin can significantly reduce this compound exposure through a drug-drug interaction [, ]. Rifampin is a potent inducer of P-glycoproteins, which are efflux transporters that can actively remove drugs from cells []. It is believed that rifampin induces the expression of these pumps, leading to increased efflux and decreased plasma levels of this compound []. This interaction highlights the importance of therapeutic drug monitoring and potential dose adjustments when combining these antibiotics.
Q10: What is the in vitro activity of this compound against different Staphylococcus aureus strains?
A10: this compound demonstrates excellent in vitro activity against various Staphylococcus aureus strains, including methicillin-resistant S. aureus (MRSA) [, , , , , ]. It exhibits MIC values ranging from 0.5 to 4 µg/mL against both methicillin-resistant and methicillin-susceptible strains [].
Q11: Has this compound shown efficacy in treating MRSA infections in animal models?
A11: Yes, studies using a guinea pig model of foreign-body infection have shown that this compound, particularly in combination with rifampin, effectively reduces bacterial load and improves cure rates compared to monotherapy [].
Q12: What are the findings from clinical trials comparing this compound to vancomycin in treating MRSA infections?
A12: Several studies have compared the effectiveness of this compound and vancomycin in treating MRSA infections.
- One large retrospective cohort study involving veterans with MRSA infections found that this compound therapy was associated with a shorter length of stay compared to vancomycin therapy [].
- Another study focusing on MRSA ventilator-associated pneumonia (VAP) observed a trend toward higher cure rates with this compound compared to vancomycin [].
Q13: What are the known mechanisms of resistance to this compound?
A13: Resistance to this compound can arise through several mechanisms, primarily affecting the drug's binding site on the ribosome [].
Q14: What are the main safety concerns associated with this compound use?
A14: While generally well-tolerated, this compound has been associated with several adverse effects, with hematological toxicity, particularly thrombocytopenia, being a significant concern [, , , , , ]. The risk of thrombocytopenia appears to be dose- and duration-dependent and is more pronounced in patients with pre-existing renal impairment or those receiving prolonged treatment [, , , ].
Q15: Are there any specific patient populations that require closer monitoring for this compound-induced thrombocytopenia?
A15: Yes, patients with pre-existing renal insufficiency, low baseline platelet counts, and those receiving prolonged this compound therapy require close monitoring for thrombocytopenia [, , ]. Additionally, patients with bacteremia and low baseline hemoglobin levels may also be at increased risk [].
Q16: Does this compound effectively penetrate the central nervous system?
A16: Yes, this compound demonstrates good penetration into the cerebrospinal fluid (CSF) []. Studies in neurosurgical patients have shown that this compound achieves adequate concentrations in the CSF, supporting its use in treating central nervous system infections caused by susceptible Gram-positive pathogens [].
Q17: What analytical methods are commonly employed to quantify this compound concentrations?
A17: High-performance liquid chromatography (HPLC) is a widely used technique for quantifying this compound concentrations in various biological matrices, including serum and CSF []. Additionally, UV-Spectrophotometric methods have been developed and validated for determining this compound content in bulk materials and tablet formulations [].
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.
![N-methyl-N-[3-(3-methyl[1,2,4]triazolo[4,3-b]pyridazin-6-yl)phenyl]acetamide](/img/structure/B1675408.png)