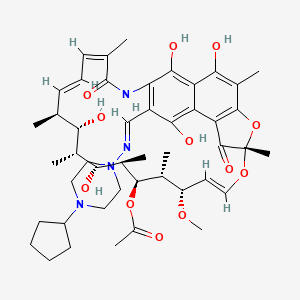
Rifapentine
- Click on QUICK INQUIRY to receive a quote from our team of experts.
- With the quality product at a COMPETITIVE price, you can focus more on your research.
Overview
Description
Rifapentine, sold under the brand name Priftin, is an antibiotic used primarily in the treatment of tuberculosis. It belongs to the rifamycin class of antibiotics and is known for its ability to inhibit bacterial RNA synthesis by targeting DNA-dependent RNA polymerase . This compound is particularly effective against Mycobacterium tuberculosis, the bacterium responsible for tuberculosis .
Mechanism of Action
Target of Action
Rifapentine, an antibiotic agent used in the treatment of pulmonary tuberculosis, primarily targets the DNA-dependent RNA polymerase in susceptible cells . This enzyme plays a crucial role in the transcription process, where it catalyzes the synthesis of RNA from DNA.
Mode of Action
It acts via the inhibition of DNA-dependent RNA polymerase, leading to a suppression of RNA synthesis and cell death . This interaction disrupts the transcription process, preventing the bacteria from synthesizing essential proteins, thereby inhibiting their growth and proliferation .
Biochemical Pathways
This compound affects the transcription pathway in bacteria. By inhibiting the DNA-dependent RNA polymerase, it disrupts the conversion of DNA into RNA, a critical step in protein synthesis . This disruption affects downstream processes, including protein synthesis and subsequent cellular functions, ultimately leading to bacterial cell death .
Pharmacokinetics
This compound exhibits certain ADME (Absorption, Distribution, Metabolism, and Excretion) properties that impact its bioavailability. It is well absorbed when taken orally and is distributed widely in body tissues and fluids, including the cerebrospinal fluid . It undergoes hepatic metabolism, where it is hydrolyzed by an esterase enzyme to form the active metabolite 25-desacetyl this compound . The drug is primarily excreted in the feces (70%), with a smaller portion excreted in the urine (17%, primarily as metabolites) . The time to peak serum concentration is between 3 to 10 hours, and the elimination half-life of this compound is approximately 17 hours .
Result of Action
The molecular and cellular effects of this compound’s action result in the suppression of RNA synthesis, leading to cell death . It has shown higher bacteriostatic and bactericidal activities, especially against intracellular bacteria growing in human monocyte-derived macrophages . It is bactericidal and has a very broad spectrum of activity against most gram-positive and gram-negative organisms, including Mycobacterium tuberculosis .
Action Environment
Environmental factors can influence the action, efficacy, and stability of this compound. For instance, the presence of food can increase the absorption of this compound, enhancing its bioavailability . Additionally, this compound’s efficacy can be influenced by the patient’s age, with clearance decreasing with increasing age . Furthermore, this compound’s action can be affected by the presence of other drugs, as it is known to induce the cytochrome P450 enzyme system, potentially leading to reduced bioavailability and enhanced clearance of some coadministered medications .
Biochemical Analysis
Biochemical Properties
Rifapentine inhibits DNA-dependent RNA polymerase activity in susceptible cells . Specifically, it interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme . This interaction is crucial for its role in biochemical reactions.
Cellular Effects
This compound has shown higher bacteriostatic and bactericidal activities especially against intracellular bacteria growing in human monocyte-derived macrophages . It influences cell function by suppressing RNA synthesis, leading to cell death .
Molecular Mechanism
The molecular mechanism of this compound involves the inhibition of DNA-dependent RNA polymerase, leading to a suppression of RNA synthesis and cell death . It exerts its effects at the molecular level through binding interactions with biomolecules, specifically bacterial RNA polymerase .
Temporal Effects in Laboratory Settings
Stability concerns have been raised for this compound stored under certain conditions .
Metabolic Pathways
This compound is metabolized in the liver and eliminated in bile and, to a much lesser extent, in urine
Transport and Distribution
This compound is well absorbed when taken orally and is distributed widely in body tissues and fluids, including the cerebrospinal fluid
Preparation Methods
Synthetic Routes and Reaction Conditions: Rifapentine is synthesized starting from rifamycin S. The process involves several key steps:
Conversion to Intermediate: Rifamycin S reacts with dihydroxy methyl tert-butyl amine in an organic solvent to form an intermediate called rifaoxazine.
Hydrolysis and Ring Opening: The intermediate undergoes hydrolysis and ring opening in the presence of a catalyst and a reductant in a second organic solvent.
Condensation: The hydrolysis product is then condensed with 1-amino-4-cyclopentyl piperazine to form this compound.
Industrial Production Methods: The industrial production of this compound involves scaling up the laboratory synthesis process. Key challenges include maintaining the purity and uniformity of the product, optimizing reaction conditions, and ensuring efficient crystallization and drying processes .
Chemical Reactions Analysis
Types of Reactions: Rifapentine undergoes several types of chemical reactions, including:
Reduction: Reduction reactions can modify the functional groups on the this compound molecule.
Substitution: Substitution reactions can occur, particularly at the piperazine ring, leading to the formation of different derivatives.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are often used.
Substitution: Substitution reactions typically involve nucleophiles like amines and alcohols.
Major Products: The major products formed from these reactions include various oxidized, reduced, and substituted derivatives of this compound .
Scientific Research Applications
Rifapentine has a wide range of scientific research applications:
Comparison with Similar Compounds
Rifampin: Commonly used in combination therapy for tuberculosis.
Rifabutin: Used primarily in the treatment of Mycobacterium avium complex infections.
Rifapentine’s unique pharmacokinetic properties and its efficacy in shorter treatment regimens make it a valuable addition to the arsenal against tuberculosis .
Properties
| Rifapentine has shown higher bacteriostatic and bactericidal activities especially against intracellular bacteria growing in human monocyte-derived macrophages. Rifapentine inhibits DNA-dependent RNA polymerase in susceptible strains of M. tuberculosis. Rifapentine acts via the inhibition of DNA-dependent RNA polymerase, leading to a suppression of RNA synthesis and cell death. | |
CAS No. |
61379-65-5 |
Molecular Formula |
C47H64N4O12 |
Molecular Weight |
877.0 g/mol |
IUPAC Name |
[(7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-26-[(4-cyclopentylpiperazin-1-yl)iminomethyl]-2,15,17,27,29-pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.14,7.05,28]triaconta-1(29),2,4,9,19,21,25,27-octaen-13-yl] acetate |
InChI |
InChI=1S/C47H64N4O12/c1-24-13-12-14-25(2)46(59)49-37-32(23-48-51-20-18-50(19-21-51)31-15-10-11-16-31)41(56)34-35(42(37)57)40(55)29(6)44-36(34)45(58)47(8,63-44)61-22-17-33(60-9)26(3)43(62-30(7)52)28(5)39(54)27(4)38(24)53/h12-14,17,22-24,26-28,31,33,38-39,43,53-57H,10-11,15-16,18-21H2,1-9H3,(H,49,59)/b13-12+,22-17+,25-14-,48-23?/t24-,26+,27+,28+,33-,38-,39+,43+,47-/m0/s1 |
InChI Key |
WDZCUPBHRAEYDL-LYDPARFQSA-N |
SMILES |
CC1C=CC=C(C(=O)NC2=C(C(=C3C(=C2O)C(=C(C4=C3C(=O)C(O4)(OC=CC(C(C(C(C(C(C1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)C=NN5CCN(CC5)C6CCCC6)C |
Isomeric SMILES |
C[C@H]1/C=C/C=C(\C(=O)NC2=C(C(=C3C(=C2O)C(=C(C4=C3C(=O)[C@](O4)(O/C=C/[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)C=NN5CCN(CC5)C6CCCC6)/C |
Canonical SMILES |
CC1C=CC=C(C(=O)NC2=C(C(=C3C(=C2O)C(=C(C4=C3C(=O)C(O4)(OC=CC(C(C(C(C(C(C1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)C=NN5CCN(CC5)C6CCCC6)C |
Appearance |
Solid powder |
Purity |
>98% (or refer to the Certificate of Analysis) |
shelf_life |
>3 years if stored properly |
solubility |
Soluble in DMSO |
storage |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Synonyms |
Rifapentine; DL 473; DL-473; DL473; R 773; R-773; R773; |
Origin of Product |
United States |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.



![5-(3,3-Dimethylbut-1-ynyl)-3-[[4-hydroxy-4-(oxolan-3-yloxymethyl)cyclohexyl]-(4-methylcyclohex-3-ene-1-carbonyl)amino]thiophene-2-carboxylic acid](/img/structure/B610407.png)
![[(1S,2S,3S,4S,5R,6S,8S,9R,13S,16S,17S)-11-ethyl-3,8,9-trihydroxy-4,6,16-trimethoxy-11-azahexacyclo[7.7.2.12,5.01,10.03,8.013,17]nonadecan-13-yl] 2-acetamidobenzoate](/img/structure/B610416.png)