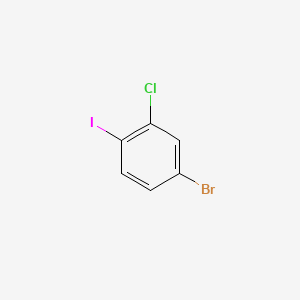
4-Bromo-2-chloro-1-iodobenzene
Cat. No. B1333647
M. Wt: 317.35 g/mol
InChI Key: OHHKQBZOURGNLR-UHFFFAOYSA-N
Attention: For research use only. Not for human or veterinary use.
Patent
US08383832B2
Procedure details


To a stirred mixture of 6-amino-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid methyl ester (6) (8.00 g, 34.16 mmol) and cesium carbonate (22.48 g, 68.31 mmol) in anhydrous anisole (76 mL) under nitrogen was added 4-bromo-2-chloroiodobenzene (1.60 g, 1.10 equiv., 4.88 mmol). The preformed catalyst, as prepared above, was then added to the mixture to provide a dark brown suspension, which was heated at 100±2° C., with stirring at 350 rpm. The reaction was monitored by HPLC analysis. After 41 hours, no 6-amino-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid methyl ester (6) remained. The reaction mixture was cooled to about 80° C. and 1M sulfuric acid (40.99 mL 40.99 mmol) was added. Gas evolution was observed after 10 minutes and the rate of addition was controlled to moderate the effervescence. At the end of the addition the pH was between 7 and 8. Additional sulfuric acid (1M, 10.25 mL, 10.25 mmol) was then added to give mobile slurry with a pH of 0. The mixture was diluted with anisole (20 mL) and Celatom FW-14 filter agent was added. It was then filtered at about 80° C. through a water-wet pad of Celatom FW-14 filter agent and the cake was washed with anisole (1×40 mL+3×20 mL), then water (10 mL). The lower aqueous layer was separated and discarded and the organic layer was washed with 10% aqueous NaCl solution (2×40 mL). This was added to a sodium hydroxide (5.46 g, 68.3 mmol) in methanol (24 mL) and the mixture was heated at 65° C. with stirring. After 17.5 hours HPLC analysis indicated that the hydrolysis of the ester was complete and the slurry was cooled to 15° C., then filtered on a sinter. The solid was washed with water (4×24 mL), MTBE (24 mL), and acetonitrile (2×25 mL) and then dried at 45° C. in a vacuum oven to provide 11.07 g of 6-(4-bromo-2-chlorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (7) as a fine pale brown solid (assay 93.7% by 1H NMR), actual wt 10.37 g (72.2% yield). 1H NMR (400 MHz, d6 DMSO) δ 3.85 (3H, s, NMe), 6.53 (1H, dd, J 9, 7, Ar—H), 7.27 (1H, dd, J 9, 2.5, Ar—H), 7.56 (1H, d, J 9, Ar—H), 7.97 (1H, s, Ar—H), 8.20 (1H, s, Ar—H), 11.5 (1H, s, CO2H). 13C NMR (100 MHz, d6 DMSO) δ 31 (CH3), 108 (CH, d, J 2), 109 (CH), 117 (C, d, J 6), 122 (C), 124 (C, d, J 7), 127 (C), 130 (C), 131 (C), 132 (C, d, J 9), 133 (C, d, J 11), 141 (C), 145 (CF, d, J 252), 146 (CH), 170 (C═O).

Quantity
8 g
Type
reactant
Reaction Step Two

Name
cesium carbonate
Quantity
22.48 g
Type
reactant
Reaction Step Two



Quantity
0 (± 1) mol
Type
reactant
Reaction Step Four





[Compound]
Name
ester
Quantity
0 (± 1) mol
Type
reactant
Reaction Step Eight

Identifiers


|
REACTION_CXSMILES
|
C[O:2][C:3]([C:5]1[C:14]([NH2:15])=[C:13]([F:16])[C:8]2[N:9]=[CH:10][N:11]([CH3:12])[C:7]=2[CH:6]=1)=[O:4].C(=O)([O-])[O-].[Cs+].[Cs+].[Br:23][C:24]1[CH:29]=[CH:28][C:27](I)=[C:26]([Cl:31])[CH:25]=1.S(=O)(=O)(O)O.[OH-].[Na+]>C1(OC)C=CC=CC=1.CO>[Br:23][C:24]1[CH:29]=[CH:28][C:27]([NH:15][C:14]2[C:5]([C:3]([OH:2])=[O:4])=[CH:6][C:7]3[N:11]([CH3:12])[CH:10]=[N:9][C:8]=3[C:13]=2[F:16])=[C:26]([Cl:31])[CH:25]=1 |f:1.2.3,6.7|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
20 mL
|
|
Type
|
solvent
|
|
Smiles
|
C1(=CC=CC=C1)OC
|
Step Two
|
Name
|
|
|
Quantity
|
8 g
|
|
Type
|
reactant
|
|
Smiles
|
COC(=O)C1=CC2=C(N=CN2C)C(=C1N)F
|
|
Name
|
cesium carbonate
|
|
Quantity
|
22.48 g
|
|
Type
|
reactant
|
|
Smiles
|
C([O-])([O-])=O.[Cs+].[Cs+]
|
|
Name
|
|
|
Quantity
|
76 mL
|
|
Type
|
solvent
|
|
Smiles
|
C1(=CC=CC=C1)OC
|
Step Three
|
Name
|
|
|
Quantity
|
1.6 g
|
|
Type
|
reactant
|
|
Smiles
|
BrC1=CC(=C(C=C1)I)Cl
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
COC(=O)C1=CC2=C(N=CN2C)C(=C1N)F
|
Step Five
|
Name
|
|
|
Quantity
|
40.99 mL
|
|
Type
|
reactant
|
|
Smiles
|
S(O)(O)(=O)=O
|
Step Six
|
Name
|
|
|
Quantity
|
10.25 mL
|
|
Type
|
reactant
|
|
Smiles
|
S(O)(O)(=O)=O
|
Step Seven
|
Name
|
|
|
Quantity
|
5.46 g
|
|
Type
|
reactant
|
|
Smiles
|
[OH-].[Na+]
|
|
Name
|
|
|
Quantity
|
24 mL
|
|
Type
|
solvent
|
|
Smiles
|
CO
|
Step Eight
[Compound]
|
Name
|
ester
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
|
Conditions


Temperature
|
Control Type
|
UNSPECIFIED
|
|
Setpoint
|
100 (± 2) °C
|
Stirring
|
Type
|
CUSTOM
|
|
Details
|
with stirring at 350 rpm
|
|
Rate
|
UNSPECIFIED
|
|
RPM
|
0
|
Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The preformed catalyst, as prepared above,
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
was then added to the mixture
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
to provide a dark brown suspension, which
|
TEMPERATURE
|
Type
|
TEMPERATURE
|
|
Details
|
The reaction mixture was cooled to about 80° C.
|
WAIT
|
Type
|
WAIT
|
|
Details
|
Gas evolution was observed after 10 minutes
|
|
Duration
|
10 min
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
the rate of addition
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
At the end of the addition the pH was between 7 and 8
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
to give mobile slurry with a pH of 0
|
FILTRATION
|
Type
|
FILTRATION
|
|
Details
|
filter agent
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
was added
|
FILTRATION
|
Type
|
FILTRATION
|
|
Details
|
It was then filtered at about 80° C. through a water-wet pad of Celatom FW-14
|
FILTRATION
|
Type
|
FILTRATION
|
|
Details
|
filter agent
|
WASH
|
Type
|
WASH
|
|
Details
|
the cake was washed with anisole (1×40 mL+3×20 mL)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The lower aqueous layer was separated
|
WASH
|
Type
|
WASH
|
|
Details
|
the organic layer was washed with 10% aqueous NaCl solution (2×40 mL)
|
TEMPERATURE
|
Type
|
TEMPERATURE
|
|
Details
|
the mixture was heated at 65° C.
|
STIRRING
|
Type
|
STIRRING
|
|
Details
|
with stirring
|
TEMPERATURE
|
Type
|
TEMPERATURE
|
|
Details
|
the slurry was cooled to 15° C.
|
FILTRATION
|
Type
|
FILTRATION
|
|
Details
|
filtered on a sinter
|
WASH
|
Type
|
WASH
|
|
Details
|
The solid was washed with water (4×24 mL), MTBE (24 mL), and acetonitrile (2×25 mL)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
dried at 45° C. in a vacuum oven
|
Outcomes


Product
Details
Reaction Time |
41 h |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
BrC1=CC(=C(C=C1)NC=1C(=CC2=C(N=CN2C)C1F)C(=O)O)Cl
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| AMOUNT: MASS | 11.07 g | |
| YIELD: PERCENTYIELD | 72.2% | |
| YIELD: CALCULATEDPERCENTYIELD | 569.1% |
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
