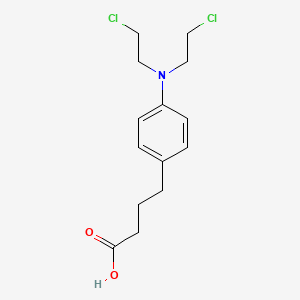
苯丁酸氮芥
描述
作用机制
氯芥胆碱通过交联DNA链发挥作用,从而阻止DNA复制和转录 . 这会导致快速分裂的癌细胞的细胞周期停滞和凋亡 . 主要分子靶点是DNA中的鸟嘌呤碱基,氯芥胆碱在那里形成共价键 . 这种机制破坏了DNA的正常功能,导致细胞死亡 .
类似化合物:
美法仑: 另一种氮芥烷化剂,用于治疗多发性骨髓瘤和卵巢癌.
环磷酰胺: 一种广泛使用的烷化剂,在各种癌症和自身免疫性疾病中都有应用.
伊福斯酰胺: 与环磷酰胺类似,但代谢途径不同,用于不同的癌症类型.
氯芥胆碱的独特性: 氯芥胆碱因其相对温和的副作用和口服给药途径而独树一帜,使患者更容易使用 . 此外,其独特的烷化模式与其他烷化剂相比提供了独特的作用机制 .
科学研究应用
氯芥胆碱具有广泛的科学研究应用:
生化分析
Biochemical Properties
Chlorambucil interacts with various biomolecules, primarily DNA, to exert its effects . It forms covalent bonds with DNA, leading to the formation of mono- or bifunctional adducts . These adducts interfere with gene expression and promote mismatched base-pairing, while bifunctional alkylation creates intra- and interstrand cross-links that inhibit DNA synthesis and cause double-strand breaks .
Cellular Effects
Chlorambucil has profound effects on various types of cells and cellular processes. It interferes with DNA replication and damages the DNA in a cell . This DNA damage induces cell cycle arrest and cellular apoptosis via the accumulation of cytosolic p53 and subsequent activation of Bcl-2-associated X protein, an apoptosis promoter .
Molecular Mechanism
Chlorambucil exerts its effects at the molecular level by blocking the formation of DNA and RNA . It alkylates and cross-links DNA during all phases of the cell cycle, inducing DNA damage via three different methods of covalent adduct generation with double-helical DNA .
Temporal Effects in Laboratory Settings
Chlorambucil demonstrates a monophasic disappearance from plasma, with a half-life of 26 minutes . The bioavailability of Chlorambucil decreases further when 4-day treatment cycles are repeated .
Dosage Effects in Animal Models
In animal models, the effects of Chlorambucil vary with different dosages . For instance, in dogs, the dosage ranges from 2-6 mg/m2, depending on the condition being treated . In cats, the dosage is typically 2 mg/m2 every 48 hours or 20 mg/m2 every two weeks .
Metabolic Pathways
Chlorambucil is extensively metabolized in the liver primarily to phenylacetic acid mustard . The pharmacokinetic data suggests that oral Chlorambucil undergoes rapid gastrointestinal absorption and plasma clearance and that it is almost completely metabolized, having extremely low urinary excretion .
Transport and Distribution
Chlorambucil is given orally and is rapidly absorbed in the gastrointestinal tract . It is extensively metabolized in the liver, primarily to phenylacetic acid mustard . The pharmacokinetic data suggests that Chlorambucil undergoes rapid gastrointestinal absorption and plasma clearance .
Subcellular Localization
Chlorambucil localizes to cancer cell mitochondria where it acts on mtDNA to arrest cell cycle and induce cell death . This localization to the mitochondria is significant as it enhances the cell-killing effect of Chlorambucil in a panel of breast and pancreatic cancer cell lines .
准备方法
合成路线和反应条件: 氯芥胆碱通过从4-氨基苯丁酸开始的多步过程合成。 反应条件通常包括使用二氯甲烷等溶剂和三乙胺等催化剂来促进烷化反应 .
工业生产方法: 在工业环境中,氯芥胆碱的生产涉及使用类似反应途径的大规模合成,但针对更高的产量和纯度进行了优化。 该过程包括严格的纯化步骤,例如重结晶和色谱法,以确保最终产品符合药物标准 .
化学反应分析
反应类型: 氯芥胆碱经历多种类型的化学反应,包括:
常用试剂和条件:
氧化: 常用的氧化剂包括过氧化氢和高锰酸钾.
取代: 硫醇和胺等亲核试剂通常用于取代反应.
主要形成的产物: 从这些反应中形成的主要产物包括DNA加合物,它们是该化合物抗癌活性的原因 .
相似化合物的比较
Melphalan: Another nitrogen mustard alkylating agent used in the treatment of multiple myeloma and ovarian cancer.
Cyclophosphamide: A widely used alkylating agent with applications in various cancers and autoimmune diseases.
Ifosfamide: Similar to cyclophosphamide but with a different metabolic pathway and used in different cancer types.
Uniqueness of Chlorambucil: Chlorambucil is unique due to its relatively mild side effect profile and oral administration route, making it more convenient for patients . Additionally, its specific alkylation pattern provides a distinct mechanism of action compared to other alkylating agents .
属性
IUPAC Name |
4-[4-[bis(2-chloroethyl)amino]phenyl]butanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H19Cl2NO2/c15-8-10-17(11-9-16)13-6-4-12(5-7-13)2-1-3-14(18)19/h4-7H,1-3,8-11H2,(H,18,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JCKYGMPEJWAADB-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CC(=CC=C1CCCC(=O)O)N(CCCl)CCCl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H19Cl2NO2 | |
| Record name | CHLORAMBUCIL | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/16180 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7020263 | |
| Record name | Chlorambucil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7020263 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
304.2 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Chlorambucil appears as white to pale beige crystalline or granular powder with a slight odor. Melting point 65-69 °C., Solid | |
| Record name | CHLORAMBUCIL | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/16180 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorambucil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014436 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>45.6 [ug/mL] (The mean of the results at pH 7.4), less than 0.1 mg/mL at 72 °F (NTP, 1992), Insoluble in water ... The sodium salt is soluble in water., The free acid is soluble at 20 °C in 1.5 parts ethanol, 2 parts acetone, 2.5 parts chloroform and 2 parts ethyl acetate; soluble in benzene and ether. Readily soluble in acid or alkali., 7.73e-02 g/L | |
| Record name | SID855863 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | CHLORAMBUCIL | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/16180 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorambucil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00291 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CHLORAMBUCIL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorambucil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014436 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Alkylating agents work by three different mechanisms: 1) attachment of alkyl groups to DNA bases, resulting in the DNA being fragmented by repair enzymes in their attempts to replace the alkylated bases, preventing DNA synthesis and RNA transcription from the affected DNA, 2) DNA damage via the formation of cross-links (bonds between atoms in the DNA) which prevents DNA from being separated for synthesis or transcription, and 3) the induction of mispairing of the nucleotides leading to mutations., As an alkylating agent, chlorambucil interferes with DNA replication and transcription of RNA, and ultimately results in the disruption of nucleic acid function. In vitro studies have shown that the major metabolite of chlorambucil (phenylacetic acid mustard), which is also a bifunctional alkylating compound, has antineoplastic activity against some neoplastic human cell lines that is approximately equal to that of chlorambucil. Therefore, the major metabolite of chlorambucil may contribute to the in vivo antitumor activity of the drug. Chlorambucil also possesses some immunosuppressive activity, principally due to its suppression of lymphocytes. The drug is the slowest acting and generally least toxic of the presently available nitrogen mustard derivatives., A marked transient increase was observed in ribonucleotide reductase activity within 2 hr of exposing BALB/c 3T3 mouse cells to DNA damaging concentrations of chlorambucil. Elevations in activity were accompanied by transient increases in the mRNA levels of both genes (R1 and R2) that code for ribonucleotide reductase. Only the protein for the limiting component for enzyme activity R2 was significantly elevated in chlorambucil treated cultures. The chlorambucil effects upon activity and regulation of ribonucleotide reductase occurred without any detectable changes in the rate of DNA synthesis, as would be expected if the elevation in enzyme activity is required for DNA repair. The chlorambucil-induced elevations in R1 and R2 message levels were blocked by treatment of cells with actinomycin D or the tumor promoter 12-O-tetradecanoylphorbol-13-acetate indicating the importance of the reductase transcriptional process in responding to the action of chlorambucil and providing evidence for the involvement of a protein kinase C pathway in the regulation of mammalian ribonucleotide reductase. In addition to the chlorambucil-induced elevations in enzyme activity, message, and protein levels, the drug was also shown to be an inhibitor of ribonucleotide reductase activity in cell-free preparations. Both R1 and R2 proteins were targets for chlorambucil, in keeping with the known alkylating abilities of the drug., /ALTERNATIVE and IN VITRO TESTS/ Reaction of one of the two chloroethyl groups of chlorambucil with the N7 position of guanine or adenine of double-stranded DNA leads to the formation of mono-adducts. These are repaired rapidly in an error-free fashion by methylguanine methyltransferase (sometimes called alkylguanine alkyltransferase). However, some cells lack this repair activity, usually because of silencing of the corresponding gene, and the unrepaired DNA mono-adduct then forms a complex with mismatch-repair enzymes. The subsequent inhibition of DNA replication can eventually induce DNA breakage. The second chloroethyl group of the DNA mono-adduct with chlorambucil can interact with proteins but more importantly, because of its juxtaposition to other bases in the major groove of DNA, it can react with a DNA base to form an interstrand DNA cross-link. This DNA crosslink complex is quite stable, and its repair requires nucleotide excision repair factors (such as xeroderma pigmentosum complementation group F-excison repair cross-complementing rodent repair deficiency, complementation group, 1-XPF-ERCC1) that act slowly by homologous recombination. The DNA cross-link attracts several binding proteins, probably the BRCA1 and BRCA2 proteins, Fanconi anemia gene product, and Nijmegen breakage syndrome gene product to form a complex. As shown in cultured HeLa cells, addition of chlorambucil prolongs S-phase and induces a corresponding mitotic delay. The magnitude of these effects correlates with the level of DNA cross-links. Treatment of cells in the G2-phase of the cell cycle does not induce mitotic delay but does inhibit DNA synthesis in the subsequent cell cycle, and causes a delay in the next mitosis, suggesting that at least some lesions induced by chlorambucil are long-lasting. | |
| Record name | Chlorambucil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00291 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CHLORAMBUCIL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Off-white, slightly granular powder, Flattened needles from petroleum ether, Fine white crystals | |
CAS No. |
305-03-3 | |
| Record name | CHLORAMBUCIL | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/16180 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorambucil | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=305-03-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Chlorambucil [USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000305033 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Chlorambucil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00291 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | chlorambucil | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=756674 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | chlorambucil | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=3088 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Chlorambucil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7020263 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Chlorambucil | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.005.603 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CHLORAMBUCIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/18D0SL7309 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CHLORAMBUCIL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorambucil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014436 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
147 to 151 °F (NTP, 1992), 65 °C | |
| Record name | CHLORAMBUCIL | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/16180 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorambucil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00291 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CHLORAMBUCIL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorambucil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014436 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: Chlorambucil is a bifunctional alkylating agent that primarily targets DNA within cells. [] It covalently binds to DNA, forming interstrand and intrastrand crosslinks, which disrupts DNA replication and transcription, ultimately leading to cell death. [, , , , ] This process is particularly effective in rapidly dividing cells, explaining its efficacy against certain cancers.
A: Research suggests that chlorambucil, in combination with ibrutinib, can influence the AKT signaling pathway in mantle cell lymphoma cells. This combination leads to upregulation of caspase-3 and downregulation of BCL-2, PI3K, and p-AKT/AKT, promoting apoptosis. []
A: While chlorambucil primarily targets DNA, certain analogues show moderate specificity for AT base pairs in the minor groove of DNA. []
A: Chlorambucil (IUPAC name: 4-[4-[bis(2-chloroethyl)amino]phenyl]butanoic acid) has the molecular formula C14H19Cl2NO2 and a molecular weight of 304.22 g/mol. [, ]
ANone: The provided research focuses mainly on chlorambucil's application as a pharmaceutical compound. Therefore, information about its compatibility with various materials or its stability outside of biological contexts and formulations is limited within these studies.
ANone: The research primarily focuses on chlorambucil's role as a chemotherapeutic agent. No catalytic properties or alternative applications are discussed within these papers.
A: While the provided research does not delve into detailed computational studies, one paper mentions the use of quantitative structure-activity relationship (QSAR) models to understand the relationship between the structure of chlorambucil analogues and their DNA binding affinity. []
A: Studies on amidine analogues of chlorambucil, where the chlorambucil moiety is linked to a 5-[4-(N-alkylamidino)phenyl]-2-furancarboxamide group, demonstrated enhanced cytotoxicity against human breast cancer MCF-7 cells compared to chlorambucil alone. This increased activity correlated with stronger DNA-binding affinity and topoisomerase II inhibition. []
A: Yes, researchers have developed chlorambucil-taurocholate, a conjugate that utilizes bile acid transporters (specifically NTCP) overexpressed in hepatocellular carcinomas to deliver the drug more selectively. [] This approach aims to enhance drug accumulation within tumor cells while potentially reducing off-target effects.
A: Researchers are exploring nanoformulations to improve chlorambucil's stability and delivery. One study encapsulated chlorambucil in a long-circulating nanoemulsion modified with poly(ethylene glycol) (PEG). [] This formulation demonstrated improved pharmacokinetic parameters, including higher AUC and longer half-life, compared to a non-PEGylated nanoemulsion and chlorambucil solution.
A: A study developed self-assembled micelles using an amphiphilic chlorambucil prodrug, CLB-HDH, where chlorambucil is conjugated to 1,6-hexanediamine hydrochloride. [] These micelles demonstrated enhanced cellular internalization and improved therapeutic activity both in vitro and in vivo compared to free chlorambucil, suggesting a promising drug delivery strategy.
A: Yes, researchers have developed photoresponsive fluorescent organic nanoconjugates using squaric acid and a coumarin-chlorambucil conjugate (Sq-Cou-Cbl). [] Upon visible light irradiation, these nanoconjugates release chlorambucil in a controlled manner and generate a photoproduct with PDT activity. This dual-action system allows for self-monitored combination therapy with enhanced anticancer activity.
ANone: The research papers primarily focus on the therapeutic aspects and clinical applications of chlorambucil. Information regarding specific SHE regulations or risk minimization strategies is not included within these studies.
A: Oral bioavailability of chlorambucil can be variable and decrease with repeated treatment cycles. [] One study found that dose-corrected AUC for the first 2 hours decreased by 33% after five cycles compared to the first cycle. [] This suggests potential for accelerated metabolism and/or reduced oral bioavailability with repeated dosing.
A: One study investigating the combination of chlorambucil and fludarabine in chronic lymphocytic leukemia patients found that concomitant administration of chlorambucil limited the dose intensity of fludarabine that could be tolerated. [] This suggests potential pharmacokinetic interactions that require careful dose adjustments in combination therapy.
A: Yes, numerous studies explored chlorambucil in combination therapies. For example: * Chlorambucil + Rituximab: This combination showed superior event-free survival compared to chlorambucil alone in patients with extranodal marginal-zone B-cell lymphoma [] and MALT lymphoma. [] * Chlorambucil + Fludarabine: This combination was studied in relapsed chronic lymphocytic leukemia, but the study revealed dose-limiting toxicity. [] * Chlorambucil + Ibrutinib: This combination demonstrated a synergistic effect in inducing apoptosis in mantle cell lymphoma cell lines. []
A: One study utilized a subcutaneous colon-38 adenocarcinoma tumor mouse model and found that chlorambucil delivered in a long-circulating nanoemulsion significantly enhanced therapeutic efficacy compared to a non-PEGylated nanoemulsion and chlorambucil solution. []
A: Research suggests that the presence of p53 and ATM gene abnormalities significantly affects the sensitivity of CLL cells to these drugs. [] CLL samples with p53 abnormalities were predominantly resistant to fludarabine, while those with ATM deletions showed increased sensitivity compared to wild-type cells. []
A: Yes, studies show that the monoclonal antibody rituximab can sensitize CLL cells to both fludarabine and chlorambucil in vitro. [, ] This sensitization effect was observed regardless of p53 or ATM gene status, highlighting rituximab's potential in overcoming resistance mechanisms. []
A: While this Q&A avoids detailing side effects, it's important to acknowledge that research mentions chlorambucil's association with myelosuppression, particularly leukopenia. [, ] Additionally, one study raised concerns about the potential long-term risk of cutaneous and hematological malignancies, particularly myeloid leukemia, with prolonged chlorambucil use in rheumatoid arthritis patients. []
A: Beyond nanoemulsions and micelles, researchers are investigating the use of tumor-specific antibodies to deliver chlorambucil directly to cancer cells. One study demonstrated that chlorambucil bound to an anti-tumor antibody could be internalized by EL4 lymphoma cells via endocytosis after forming "caps" on the cell surface. [] This targeted approach aims to increase drug concentration at the tumor site and potentially minimize systemic toxicity.
A: While the research doesn't pinpoint specific biomarkers for predicting chlorambucil's efficacy, it highlights the importance of genetic and molecular factors. One study found that Binet stage C and advanced Rai stages (3 and 4) correlated with statistically significant lower progression-free survival in CLL patients treated with chlorambucil and rituximab. [] This suggests that disease stage and other prognostic indicators could play a role in treatment response.
A: High-performance liquid chromatography (HPLC) is commonly employed to measure chlorambucil and its active metabolite, phenylacetic acid mustard, in biological samples. [] This method enables researchers to analyze drug concentrations and pharmacokinetic parameters.
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。


![4-[3-Oxo-3-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-YL)-propenyl]-benzoic acid](/img/structure/B1668566.png)