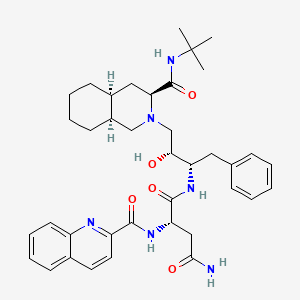
Saquinavir
Übersicht
Beschreibung
Saquinavir is an antiretroviral medication used in combination with other medications to treat or prevent HIV/AIDS. It belongs to the protease inhibitor class and works by blocking the HIV protease enzyme, which is essential for the virus to mature and replicate . This compound was the first protease inhibitor approved by the FDA in 1995 and is marketed under brand names such as Invirase and Fortovase .
Wirkmechanismus
Target of Action
Saquinavir is an HIV-1 protease inhibitor . The primary target of this compound is the HIV-1 protease , an enzyme that plays a crucial role in the life cycle of the HIV-1 virus .
Mode of Action
This compound works by inhibiting the HIV-1 protease . This protease is responsible for cleaving protein molecules into smaller fragments, a vital process for both viral replication within the cell and the release of mature viral particles from an infected cell . By blocking the action of this protease, this compound impedes the virus from maturing and replicating .
Biochemical Pathways
The inhibition of the HIV-1 protease by this compound leads to the formation of structurally defective and functionally inactive viral particles . This action disrupts the normal life cycle of the HIV virus, preventing it from replicating and spreading within the body .
Pharmacokinetics
This compound is extensively metabolized in the liver following oral administration . Over 90% of its biotransformation is mediated by the CYP3A4 isoenzyme . This compound is rapidly metabolized to a number of inactive mono- and di-hydroxylated compounds .
Result of Action
The result of this compound’s action is a decrease in the amount of HIV in the blood .
Action Environment
The action of this compound can be influenced by various environmental factors. For instance, the presence of other medications can affect its efficacy and stability. This compound is often used in combination with other antiretroviral agents for the treatment of HIV-1 . The co-administration of ritonavir, a potent enzyme inhibitor, increases the bioavailability and subsequent serum concentrations of this compound, thus dramatically improving its antiviral activity .
Wissenschaftliche Forschungsanwendungen
Saquinavir hat ein breites Spektrum an Anwendungen in der wissenschaftlichen Forschung:
Chemie: Als Modellverbindung für die Untersuchung von Proteaseinhibitoren und deren Wechselwirkungen mit Enzymen eingesetzt.
Biologie: Untersucht auf seine Auswirkungen auf die Virusreplikation und Proteinsynthese.
Medizin: In erster Linie zur Behandlung von HIV/AIDS eingesetzt.
Industrie: Bei der Entwicklung neuer antiviraler Medikamente und Therapeutika eingesetzt.
5. Wirkmechanismus
This compound übt seine antivirale Aktivität aus, indem es das HIV-1-Protease-Enzym hemmt. Dieses Enzym ist für die Spaltung von Polyproteinen in funktionelle virale Proteine entscheidend. Durch die Blockierung dieses Enzyms verhindert this compound die Reifung des Virus und führt zur Bildung nicht-infektiöser Viruspartikel . Das primäre molekulare Ziel ist die HIV-1-Protease, und der Weg beinhaltet die Störung der Virusreplikation und -assemblierung .
Ähnliche Verbindungen:
Ritonavir: Ein weiterer Proteaseinhibitor, der in Kombination mit anderen Antiretroviralen eingesetzt wird. Es ist bekannt für seine Fähigkeit, die Wirksamkeit anderer Proteaseinhibitoren zu steigern, indem es deren Stoffwechsel hemmt.
Indinavir: Ein Proteaseinhibitor, der ebenfalls die HIV-1-Protease angreift, aber unterschiedliche pharmakokinetische Eigenschaften besitzt.
Lopinavir: Oft in Kombination mit Ritonavir verwendet, hat es einen ähnlichen Wirkmechanismus, aber unterschiedliche Resistenzprofile.
Einzigartigkeit von this compound: this compound war der erste Proteaseinhibitor, der zugelassen wurde, und hat im Vergleich zu anderen antiretroviralen Therapien ein relativ mildes Nebenwirkungsprofil . Seine einzigartige Struktur und Bindungsaffinität zur HIV-1-Protease machen es zu einem wertvollen Bestandteil von Kombinationstherapien für HIV/AIDS .
Biochemische Analyse
Biochemical Properties
Saquinavir is an inhibitor of the HIV-1 protease enzyme . It interacts with this enzyme, preventing the proteolysis of the Gag polyprotein, which results in the production of immature, non-infectious viral particles . More than 90% of its biotransformation is mediated by the CYP3A4 isoenzyme .
Cellular Effects
This compound exerts its antiviral activity by inhibiting an enzyme critical for the HIV-1 viral lifecycle . It influences cell function by preventing HIV-1 protease activity, thus inhibiting the production of mature, infectious viral particles . This impacts cellular metabolism and gene expression related to viral replication.
Molecular Mechanism
The molecular mechanism of this compound involves the inhibition of the HIV-1 protease enzyme . Its design is based on the “peptidomimetic” principle, where the molecule contains a hydroxyethylene scaffold that mimics the normal peptide linkage (cleaved by HIV protease) but which itself cannot be cleaved . This prevents HIV-1 protease activity, inhibiting the proteolysis of the Gag polyprotein, and resulting in the production of immature, non-infectious viral particles .
Temporal Effects in Laboratory Settings
The effects of this compound over time in laboratory settings have been observed to change. It has been found that all fatty acid levels were lower compared with pre-treatment levels after 4 weeks of treatment, suggesting a prolonged effect of the antiretroviral drug treatment lasting beyond the 4 week post-treatment observation period .
Dosage Effects in Animal Models
In animal models, the effects of this compound vary with different dosages. The oral LD50 of this compound in both rats and mice is >5 g/kg . No acute toxicities or sequelae were noted in a patient ingesting 8 grams of this compound as a single dose .
Metabolic Pathways
This compound is extensively metabolized in the liver following oral administration . In vitro studies have shown that more than 90% of its biotransformation is mediated by the CYP3A4 isoenzyme . This compound is rapidly metabolized to a number of inactive mono- and di-hydroxylated compounds .
Transport and Distribution
This compound is transported by multidrug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporter (MRP2) . The steady-state volume of distribution of this compound is approximately 700 L, suggesting extensive distribution into tissues .
Subcellular Localization
Given its role as a protease inhibitor and its extensive distribution into tissues , it can be inferred that this compound likely localizes to the sites of viral protein synthesis and assembly within the cell
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: The synthesis of Saquinavir involves multiple steps, starting with the preparation of key intermediates. One method involves the methyl sulfonylation of (1S, 2S)-(1-benzyl-3-chloro-2-hydroxypropyl) tert-butyl carbamate using methylsulfonyl chloride in the presence of triethylamine and methylbenzene under a nitrogen atmosphere. This intermediate is then reacted with metallic acetate in the presence of 18-crown ether-6 and methylbenzene to obtain (1S, 2R)-(1-benzyl-3-chloro-2-acetic acid propyl) tert-butyl carbamate. Further reactions with KOH, THF, and ethanol yield (2R, 3S)-1,2-epoxy-3-tert-butyloxycarboryl amino-4-phenyl butane .
Industrial Production Methods: Industrial production of this compound typically involves large-scale synthesis using optimized reaction conditions to ensure high yield and purity. The process includes the use of advanced techniques such as differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and Fourier transform infrared spectroscopy (FT-IR) for characterization and quality control .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Saquinavir unterliegt verschiedenen chemischen Reaktionen, einschließlich Oxidation, Reduktion und Substitution. Diese Reaktionen sind entscheidend für seinen Stoffwechsel und seine Ausscheidung aus dem Körper.
Häufige Reagenzien und Bedingungen:
Oxidation: this compound kann unter Verwendung von Reagenzien wie Wasserstoffperoxid oder Kaliumpermanganat oxidiert werden.
Reduktion: Reduktionsreaktionen können Agenzien wie Natriumborhydrid oder Lithiumaluminiumhydrid beinhalten.
Substitution: Substitutionsreaktionen verwenden häufig Halogenierungsmittel wie Thionylchlorid oder Phosphortribromid.
Hauptprodukte: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, umfassen verschiedene Metaboliten, die hauptsächlich über Stuhl und Urin ausgeschieden werden .
Vergleich Mit ähnlichen Verbindungen
Ritonavir: Another protease inhibitor used in combination with other antiretrovirals. It is known for its ability to boost the effectiveness of other protease inhibitors by inhibiting their metabolism.
Indinavir: A protease inhibitor that also targets the HIV-1 protease but has different pharmacokinetic properties.
Lopinavir: Often used in combination with Ritonavir, it has a similar mechanism of action but different resistance profiles.
Uniqueness of Saquinavir: this compound was the first protease inhibitor to be approved and has a relatively benign adverse effect profile compared to other antiretroviral therapies . Its unique structure and binding affinity to the HIV-1 protease make it a valuable component of combination therapies for HIV/AIDS .
Eigenschaften
IUPAC Name |
(2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarbamoyl)-3,4,4a,5,6,7,8,8a-octahydro-1H-isoquinolin-2-yl]-3-hydroxy-1-phenylbutan-2-yl]-2-(quinoline-2-carbonylamino)butanediamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
QWAXKHKRTORLEM-UGJKXSETSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)(C)NC(=O)C1CC2CCCCC2CN1CC(C(CC3=CC=CC=C3)NC(=O)C(CC(=O)N)NC(=O)C4=NC5=CC=CC=C5C=C4)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@H]([C@H](CC3=CC=CC=C3)NC(=O)[C@H](CC(=O)N)NC(=O)C4=NC5=CC=CC=C5C=C4)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C38H50N6O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
149845-06-7 (monomethanesulfonate (salt)) | |
| Record name | Saquinavir [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0127779208 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID6044012 | |
| Record name | Saquinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6044012 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
670.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Insoluble, In water, 0.22 g/100 mL @ 25 °C | |
| Record name | Saquinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01232 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | SAQUINAVIR | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7161 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Vapor Pressure |
2X10-31 mm Hg @ 25 °C /Estimated/ | |
| Record name | SAQUINAVIR | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7161 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
The HIV lifecycle is comprised of 3 distinct stages: assembly, involving creation and packaging of essential viral components; budding, wherein the viral particle crosses the host cell plasma membrane and forms a lipid envelope; and maturation, wherein the viral particle alters its structure and becomes infectious. At the center of this lifecycle is the Gag polyprotein which, along with the products of its proteolysis, coordinate these stages and function as the major structural proteins of the virus. The HIV-1 protease enzyme, a dimeric aspartic protease, is the enzyme responsible for cleaving the Gag polyprotein and thus plays a critical role in many aspects of the HIV viral lifecycle. Saquinavir is an inhibitor of the HIV-1 protease enzyme. Its design is based on the "peptidomimetic" principle, wherein the molecule contains a hydroxyethylene scaffold that mimics the normal peptide linkage (cleaved by HIV protease) but which itself cannot be cleaved. By preventing HIV-1 protease activity, and thus the proteolysis of the Gag polyprotein, saquinavir results in the production of immature, non-infectious viral particles., While the complete mechanisms of antiviral activity of saquinavir have not been fully elucidated, saquinavir apparently inhibits replication of retroviruses, including human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2), by interfering with HIV protease. The drug, therefore, exerts a virustatic effect against retroviruses by acting as an HIV protease inhibitor., Saquinavir is a selective, competitive, reversible inhibitor of HIV protease. HIV protease, an aspartic endopeptidase that functions as a homodimer, plays an essential role in the replication cycle of HIV and the formation of infectious virus. During HIV replication, HIV protease cleaves viral polypeptide products of the gag and gag-pol genes (i.e., p55 and p160) to form structural proteins of the virion core (i.e., p17, p24, p9, and p7) and essential viral enzymes (i.e., reverse transcriptase, integrase, and protease). Because saquinavir is a structural analog of the HIV Phe-Pro protease cleavage site, the drug inhibits the function of the enzyme. By interfering with the formation of these essential proteins and enzymes, saquinavir blocks maturation of the virus and causes the formation of nonfunctional, immature, noninfectious virions. Saquinavir is active in both acutely and chronically infected cells since it targets the HIV replication cycle after translation and before assembly. Thus, the drug is active in chronically infected cells (e.g., monocytes and macrophages) that generally are not affected by nucleoside reverse transcriptase inhibitors (e.g., didanosine, lamivudine, stavudine, zalcitabine, zidovudine). Saquinavir does not affect early stages of the HIV replication cycle; however, the drug interferes with the production of infectious HIV and limits further infectious spread of the virus., Unlike nucleoside antiretroviral agents, the antiviral activity of saquinavir does not depend on intracellular conversion to an active metabolite. Saquinavir and other HIV protease inhibitors (e.g., amprenavir, indinavir, lopinavir, nelfinavir, ritonavir) act at a different stage of the HIV replication cycle than nucleoside and nonnucleoside reverse transcriptase inhibitors, and results of in vitro studies indicate that the antiretroviral effects of some nucleoside reverse transcriptase inhibitors and HIV protease inhibitors may be additive or synergistic. | |
| Record name | Saquinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01232 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | SAQUINAVIR | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7161 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White crystalline solid, Off-white to white very fine powder | |
CAS No. |
127779-20-8 | |
| Record name | Saquinavir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=127779-20-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Saquinavir [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0127779208 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Saquinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01232 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Saquinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6044012 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | SAQUINAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/L3JE09KZ2F | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | SAQUINAVIR | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7161 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![2-(5-Bromo-2-thienyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborine](/img/structure/B1662095.png)