Linagliptin
Übersicht
Beschreibung
Linagliptin ist ein Medikament zur Behandlung von Typ-2-Diabetes mellitus. Es ist ein Dipeptidylpeptidase-4 (DPP-4)-Inhibitor, der durch Erhöhung der Insulinproduktion und Verringerung der Glukagonproduktion in der Bauchspeicheldrüse wirkt . This compound wird oral eingenommen und unter den Markennamen Tradjenta, Trajenta und Trazenta vermarktet .
Wirkmechanismus
Target of Action
Linagliptin is a selective inhibitor of dipeptidyl peptidase-4 (DPP-4) . DPP-4 is an enzyme responsible for the degradation of incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) . These hormones play a crucial role in regulating insulin secretion and glucose homeostasis .
Mode of Action
This compound works by inhibiting the DPP-4 enzyme, thereby slowing the inactivation of GLP-1 and GIP . This leads to an increase in the levels of these active incretins, which in turn enhances the production of insulin and decreases the production of glucagon by the pancreas . The result is a reduction in blood glucose levels in a glucose-dependent manner .
Biochemical Pathways
This compound’s administration leads to a decrease in the concentration of proinflammatory factors such as TNF-α, IL-6 and increases the number of anti-inflammatory patrolling monocytes . It also affects vascular dysfunction-related reactive oxygen species and inflammation, thus exhibiting anti-oxidative and anti-inflammatory properties . Moreover, this compound has been shown to have a positive effect on metabolic dysfunction and renal and/or cardiovascular damage .
Pharmacokinetics
This compound has a unique pharmacokinetic profile characterized by target-mediated nonlinear pharmacokinetics, concentration-dependent protein binding, minimal renal clearance, and no requirements for dose adjustment for any intrinsic or extrinsic factor . It is rapidly absorbed after oral administration, with maximum plasma concentration occurring after approximately 90 minutes, and reaches steady-state concentrations within 4 days . The majority of this compound is eliminated as the parent compound, demonstrating that metabolism plays a minor role in the overall pharmacokinetics in humans . This compound is eliminated primarily in feces, with only around 5% of the oral therapeutic dose excreted in the urine at steady state .
Result of Action
The inhibition of DPP-4 by this compound leads to increased levels of active incretins, which in turn stimulate the production of insulin and inhibit the production of glucagon . This results in a reduction of blood glucose levels in a glucose-dependent manner . Additionally, this compound has been shown to have neuroprotective properties, with potential effects on tissue repair and neuroprotection .
Action Environment
The efficacy of this compound can be influenced by various environmental factors. For instance, this compound’s pharmacokinetic profile allows it to be administered without any dose adjustment in conditions of renal impairment . Furthermore, this compound’s efficacy in improving glycemic control has been demonstrated in several different studies, both as a monotherapy and as an add-on therapy . .
Wissenschaftliche Forschungsanwendungen
Linagliptin hat eine breite Palette an Anwendungen in der wissenschaftlichen Forschung, insbesondere in den Bereichen Medizin und Biologie. Es wird hauptsächlich zur Behandlung von Hyperglykämie bei Patienten mit Typ-2-Diabetes mellitus eingesetzt, indem es das DPP-4-Enzym hemmt . Darüber hinaus hat this compound neuroprotektive Eigenschaften gezeigt, was es zu einem potenziellen Kandidaten für die Behandlung neurodegenerativer Erkrankungen und zerebraler Ischämie macht . Untersuchungen haben auch seine positiven Auswirkungen auf die Gefäßfunktionen und seine Fähigkeit, Entzündungen zu reduzieren, gezeigt .
Wirkmechanismus
This compound entfaltet seine Wirkung, indem es das DPP-4-Enzym hemmt, das für den Abbau von Inkretin-Hormonen wie Glucagon-like Peptide-1 (GLP-1) und Glucose-abhängigem insulinotropen Polypeptid (GIP) verantwortlich ist . Durch die Hemmung von DPP-4 erhöht this compound die Spiegel aktiver Inkretin-Hormone, was zu einer erhöhten Insulinproduktion und einer verringerten Glukagonproduktion führt . Dies trägt zur Regulierung des Blutzuckerspiegels bei Patienten mit Typ-2-Diabetes bei .
Biochemische Analyse
Biochemical Properties
Linagliptin interacts with the enzyme DPP-4, inhibiting its activity . This inhibition slows the breakdown of incretin hormones, glucagon-like peptide-1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP), which play crucial roles in regulating insulin secretion and glucose homeostasis .
Cellular Effects
This compound has been shown to decrease the concentration of proinflammatory factors such as TNF-α, IL-6, and increase the number of anti-inflammatory patrolling monocytes CX3CR1 bright . It also improves vascular functions by increasing the production of nitric oxide (NO) and limiting the concentration of apolipoprotein B .
Molecular Mechanism
This compound is a competitive, reversible DPP-4 inhibitor . By inhibiting DPP-4, it slows the inactivation of GLP-1 and GIP, promoting blood glucose level reduction in a glucose-dependent manner .
Temporal Effects in Laboratory Settings
In clinical trials, this compound demonstrated efficacy in reducing glycated hemoglobin (HbA1c) levels in type 2 diabetes patients over time, while maintaining a placebo-like safety and tolerability profile .
Dosage Effects in Animal Models
In animal models of diabetes, this compound has shown significant reductions in plasma leptin levels and hepatic steatosis when administered at doses of 3 or 30 mg/kg .
Metabolic Pathways
This compound is involved in the incretin metabolic pathway. It inhibits the DPP-4 enzyme, slowing the breakdown of incretin hormones and thereby increasing their levels .
Transport and Distribution
This compound exhibits concentration-dependent protein binding in human plasma in vitro and has a large apparent volume of distribution, demonstrating extensive distribution into tissues .
Vorbereitungsmethoden
Linagliptin wird durch eine Reihe chemischer Reaktionen unter Verwendung von Zwischenverbindungen synthetisiert. Ein verbessertes Verfahren zur Herstellung von this compound umfasst die Reinigung der Zwischenverbindungen und deren Umwandlung in this compound . Die Synthese umfasst typischerweise Schritte wie Kondensationsreaktionen, Reinigung und Kristallisation, um eine hohe Ausbeute und Reinheit zu erzielen . Industrielle Produktionsmethoden konzentrieren sich auf die Optimierung dieser Schritte, um eine gleichbleibende Qualität und Effizienz zu gewährleisten .
Analyse Chemischer Reaktionen
Linagliptin durchläuft verschiedene chemische Reaktionen, darunter Oxidation, Reduktion und Substitution. Häufig verwendete Reagenzien in diesen Reaktionen sind Oxidationsmittel, Reduktionsmittel und Katalysatoren . Beispielsweise ist this compound besonders anfällig für den Abbau, wenn es Säure und Peroxid ausgesetzt ist, was zur Bildung von Abbauprodukten führt . Das Verständnis dieser Reaktionen ist entscheidend für die Optimierung der Formulierung und die Gewährleistung der therapeutischen Wirksamkeit .
Vergleich Mit ähnlichen Verbindungen
Linagliptin ist einer von mehreren DPP-4-Inhibitoren, die zur Behandlung von Typ-2-Diabetes eingesetzt werden. Andere ähnliche Verbindungen sind Sitagliptin, Saxagliptin und Alogliptin . Im Vergleich zu diesen Verbindungen hat this compound ein einzigartiges pharmakokinetisches Profil mit einer langen terminalen Halbwertszeit und einer überwiegend nicht-renalen Elimination . Dies ermöglicht eine einmal tägliche Dosierung ohne Notwendigkeit einer Dosisanpassung bei Patienten mit Nierenfunktionsstörungen . Darüber hinaus hat this compound eine anhaltende und maximale Hemmung der DPP-4-Aktivität gezeigt, die bei einigen anderen DPP-4-Inhibitoren nicht beobachtet wird .
Eigenschaften
IUPAC Name |
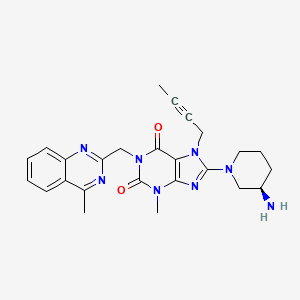
8-[(3R)-3-aminopiperidin-1-yl]-7-but-2-ynyl-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]purine-2,6-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LTXREWYXXSTFRX-QGZVFWFLSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC#CCN1C2=C(N=C1N3CCCC(C3)N)N(C(=O)N(C2=O)CC4=NC5=CC=CC=C5C(=N4)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC#CCN1C2=C(N=C1N3CCC[C@H](C3)N)N(C(=O)N(C2=O)CC4=NC5=CC=CC=C5C(=N4)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C25H28N8O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID201021653 | |
| Record name | Linagliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID201021653 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
472.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
<1 mg/mL, Soluble in methanol; sparingly soluble in ethanol; very slightly soluble in isopropanol, alcohol | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Linagliptin is a competitive, reversible DPP-4 inhibitor. Inhibition of this enzyme slows the breakdown of GLP-1 and glucose-dependant insulinotropic polypeptide (GIP). GLP-1 and GIP stimulate the release of insulin from beta cells in the pancreas while inhibiting release of glucagon from pancreatic beta cells. These effects together reduce the breakdown of glycogen in the liver and increase insulin release in response to glucose., Linagliptin is an inhibitor of DPP-4, an enzyme that degrades the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Thus, linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin in a glucose-dependent manner and decreasing the levels of glucagon in the circulation. Both incretin hormones are involved in the physiological regulation of glucose homeostasis. Incretin hormones are secreted at a low basal level throughout the day and levels rise immediately after meal intake. GLP-1 and GIP increase insulin biosynthesis and secretion from pancreatic beta-cells in the presence of normal and elevated blood glucose levels. Furthermore, GLP-1 also reduces glucagon secretion from pancreatic alpha-cells, resulting in a reduction in hepatic glucose output. | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to yellow solid; also reported as a crystalline solid | |
CAS No. |
668270-12-0 | |
| Record name | Linagliptin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=668270-12-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Linagliptin [USAN:INN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0668270120 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID201021653 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 8-[(3R)-3-aminopiperidin-1-yl]-7-but-2-ynyl-3-methyl-1-[(4-methylquinazolin-2yl)methyl]purine-2,6-dione | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LINAGLIPTIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3X29ZEJ4R2 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
190-196, 202 °C | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: Linagliptin is a highly selective inhibitor of the enzyme dipeptidyl peptidase-4 (DPP-4). [] This enzyme is responsible for the rapid degradation of incretin hormones, such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). [, , ] By inhibiting DPP-4, this compound increases the levels of these incretin hormones, which in turn stimulate insulin secretion from pancreatic beta cells and suppress glucagon secretion from alpha cells, ultimately leading to improved glycemic control. [, , , ]
A: Research suggests that this compound can improve insulin sensitivity, particularly in the liver. Studies in diet-induced obese mice showed that long-term this compound treatment improved glucose disposal rates and suppressed hepatic glucose production during a euglycemic-hyperinsulinemic clamp, indicating enhanced insulin sensitivity. []
A: The molecular formula of this compound is C25H28N8O2, and its molecular weight is 472.54 g/mol. [, ]
A: While the provided abstracts don't delve into detailed spectroscopic analysis, they do mention techniques like X-ray crystallography being used to understand this compound's interaction with the DPP-4 enzyme. [] This technique helps visualize the drug's binding mode within the enzyme's active site.
ANone: The provided abstracts focus primarily on the pharmacological aspects of this compound and do not provide information on its material compatibility or stability in various conditions beyond standard pharmaceutical formulations.
ANone: this compound itself doesn't possess catalytic properties. Its action relies on inhibiting the catalytic activity of the DPP-4 enzyme.
A: While not explicitly detailed in the provided abstracts, computational chemistry techniques like QSAR (Quantitative Structure-Activity Relationship) are likely employed in the drug discovery and optimization process of this compound. [] These methods correlate structural features with biological activity, aiding in the design of more potent and selective DPP-4 inhibitors.
A: this compound is typically formulated as oral tablets for once-daily administration. [, , ] One study explored the bioequivalence of a fixed-dose combination tablet containing this compound, empagliflozin, and extended-release metformin compared to separate tablets, aiming to improve patient adherence. []
ANone: The provided abstracts primarily focus on preclinical and clinical research findings related to this compound and do not delve into specific SHE (Safety, Health, and Environment) regulations.
A: this compound demonstrates good oral absorption. [] Studies in rats using high-resolution autoradiography revealed that after intravenous administration, this compound-related radioactivity exhibited time- and dose-dependent localization in the kidneys, liver, and small intestine, reflecting the known distribution of DPP-4. [, ]
A: Unlike many other DPP-4 inhibitors primarily cleared by the kidneys, this compound is predominantly excreted via the enterohepatic system, with a small portion eliminated renally. [, ] This characteristic allows for its use without dose adjustment in patients with renal impairment. [, , ]
A: One study investigated the effect of food on this compound bioavailability when administered as a fixed-dose combination tablet with metformin. It concluded that food did not significantly impact this compound's bioavailability. []
A: Yes, in diet-induced obese mice, this compound significantly reduced liver fat content and improved glycemic control, as evidenced by lower blood glucose levels and improved glucose tolerance. [, ]
A: Numerous clinical trials have demonstrated that this compound effectively improves glycemic control in patients with type 2 diabetes. It significantly reduces HbA1c levels when used as monotherapy or in combination with other antidiabetic drugs. [, , , , , , , , ]
A: The CAROLINA trial, a large cardiovascular outcome trial, compared this compound to glimepiride (a sulfonylurea) in patients with type 2 diabetes and established cardiovascular disease or high cardiovascular risk. The results showed that this compound was non-inferior to glimepiride in reducing the risk of cardiovascular events. []
ANone: The provided abstracts don't specifically address resistance mechanisms to this compound or cross-resistance with other DPP-4 inhibitors or drug classes.
ANone: The provided abstracts focus primarily on oral administration of this compound and don't explore alternative drug delivery systems or targeting strategies.
ANone: The research presented in the abstracts doesn't specifically investigate biomarkers for predicting this compound efficacy, monitoring treatment response, or identifying potential adverse effects.
A: While not extensively discussed in the provided abstracts, high-performance liquid chromatography (HPLC) is a widely used technique for quantifying this compound in biological samples. [, ]
ANone: The ecological impact and degradation pathways of this compound aren't discussed in the provided research abstracts.
A: A dedicated study examined the impact of varying tablet dissolution characteristics on the bioavailability of this compound in a fixed-dose combination with metformin. It concluded that the observed differences in dissolution profiles did not significantly affect the bioavailability of either drug. []
ANone: While the provided research abstracts don't elaborate on specific analytical method validation details for this compound, it's crucial to adhere to established guidelines to ensure accuracy, precision, and specificity when quantifying this drug in various matrices.
ANone: The provided abstracts don't specifically address the immunogenicity potential of this compound or any immunological responses associated with its use.
A: Research indicates that this compound is a substrate for P-glycoprotein (P-gp), a drug efflux transporter. [, ] This interaction could potentially influence its absorption and distribution, particularly in organs with high P-gp expression, such as the intestine and brain. [, ]
ANone: The abstracts primarily focus on this compound's pharmacological properties and don't delve into its biocompatibility or biodegradability aspects.
ANone: The abstracts don't provide information related to the recycling or waste management of this compound.
A: The research abstracts highlight the use of various research tools and resources, including animal models like diabetic db/db mice and ZDF rats, cell-based assays using human U937 monocytes and brain microvascular endothelial cells, and clinical trials involving diverse patient populations. [, , , , ] These resources are essential for investigating this compound's efficacy and safety.
A: The approval of this compound by the US Food and Drug Administration on May 2, 2011, marked a significant milestone in the development of DPP-4 inhibitors for treating type 2 diabetes. [, ] This approval was based on a comprehensive clinical development program involving thousands of patients and demonstrating the drug's efficacy and safety profile.
A: The research on this compound highlights the importance of interdisciplinary collaboration, involving scientists from various fields such as pharmacology, endocrinology, cardiology, and medicinal chemistry. [, , , ] For instance, understanding this compound's effects on cardiovascular outcomes requires expertise from both cardiologists and endocrinologists. Similarly, developing and optimizing the drug's formulation and delivery systems necessitate contributions from pharmaceutical scientists.
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![5-[2-(3-azidophenyl)ethyl-methylamino]-2-propan-2-yl-2-(3,4,5-trimethoxyphenyl)pentanenitrile;hydrochloride](/img/structure/B1675342.png)
![Benzamide, 4-[(3,3-dimethyl-1-oxobutyl)amino]-3,5-difluoro-N-2-thiazolyl-](/img/structure/B1675344.png)