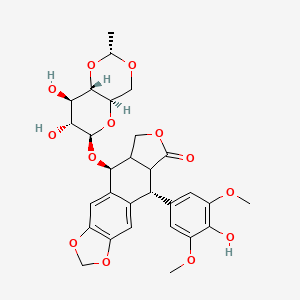
Etoposide
Übersicht
Beschreibung
Etoposide ist ein Chemotherapeutikum, das hauptsächlich zur Behandlung verschiedener Krebsarten eingesetzt wird, darunter Hodenkrebs, Lungenkrebs, Lymphom, Leukämie, Neuroblastom und Eierstockkrebs . Es ist ein semisynthetisches Derivat von Podophyllotoxin, einem Naturstoff, der aus den Wurzeln und Rhizomen von Pflanzen der Gattung Podophyllum gewonnen wird . This compound wirkt, indem es das Enzym Topoisomerase II hemmt, das für die DNA-Replikation und Zellteilung unerlässlich ist .
Herstellungsmethoden
This compound wird durch einen semisynthetischen Prozess ausgehend von Podophyllotoxin synthetisiert. Eine gängige Methode beinhaltet die direkte Kondensation von 4’-Demethylepipodophyllotoxin mit 2,3-Di-O-Dichloracetyl-(4,6-O-Ethyliden)-β-D-Glucopyranose in Gegenwart von Trimethylsilyltrifluormethansulfonat (TMSOTf) zu 4’-Demethylepipodophyllotoxin-4-(2,3-Di-O-Dichloracetyl-4,6-0-Ethyliden)-β-D-Glucopyranosid, das dann in this compound umgewandelt wird . Diese Methode bietet im Vergleich zu bestehenden Techniken höhere Ausbeuten, verkürzte Reaktionszeiten und günstigere Isolierverfahren .
Wirkmechanismus
Target of Action
Etoposide primarily targets DNA topoisomerase II , an enzyme that plays a crucial role in DNA replication and transcription . This enzyme is responsible for relieving the torsional strain in DNA by introducing transient breaks, allowing the DNA strands to pass through one another, and then resealing the breaks .
Mode of Action
This compound interacts with its target by forming a ternary complex with topoisomerase II and DNA . This complex induces breaks in double-stranded DNA and prevents repair by topoisomerase II binding . The accumulation of these breaks in DNA prevents entry into the mitotic phase of cell division, leading to cell death . This compound is cell cycle-dependent and phase-specific, affecting mainly the S and G2 phases of cell division .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the DNA damage response pathway . The drug causes DNA double-strand breaks (DSBs), which activate a series of cellular responses, including cell cycle arrest, DNA repair, and, if the damage is too severe, apoptosis . The activation of p53, a key player in this pathway, is part of a positive feedback loop that amplifies the DNA damage signal and leads to cell cycle arrest or apoptosis .
Pharmacokinetics
This compound exhibits highly variable bioavailability (25 to 75%) when administered orally . It is metabolized in the liver, primarily by the CYP3A4 enzyme . The drug’s elimination half-life varies: it’s approximately 6 hours for oral administration, 6-12 hours for intravenous administration in adults, and around 3 hours in children . This compound is excreted through both the kidneys and feces .
Result of Action
The primary molecular effect of this compound is the induction of DNA double-strand breaks , which can lead to cell cycle arrest and apoptosis . On a cellular level, this results in the death of rapidly dividing cells, particularly cancer cells . This can also affect healthy cells that divide rapidly, such as those in the bone marrow, gastrointestinal tract, and hair follicles, leading to side effects like low blood cell counts, nausea, and hair loss .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, the drug’s absorption can be affected by the pH of the stomach, and its metabolism can be influenced by the presence of other drugs that induce or inhibit CYP3A4 . Additionally, genetic variations in the target enzyme, topoisomerase II, or in drug transporters and metabolizing enzymes, can also impact the drug’s efficacy and toxicity .
Wissenschaftliche Forschungsanwendungen
Etoposide hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung, darunter:
Medizin: This compound ist ein wichtiger Bestandteil von Chemotherapie-Regimen für verschiedene Krebsarten, darunter Hodenkrebs, Lungenkrebs und Lymphom.
Wirkmechanismus
This compound entfaltet seine Wirkung, indem es das Enzym Topoisomerase II hemmt, das für die Regulierung der DNA-Topologie durch einen Doppelstrang-Passage-Mechanismus verantwortlich ist . Durch die Bildung eines Komplexes mit Topoisomerase II und DNA induziert this compound Brüche in doppelsträngiger DNA und verhindert die Reparatur durch die Bindung von Topoisomerase II . Angehäufte Brüche in der DNA verhindern den Eintritt in die mitotische Phase der Zellteilung, was zum Zelltod führt . This compound wirkt hauptsächlich in den G2- und S-Phasen des Zellzyklus .
Vorbereitungsmethoden
Etoposide is synthesized through a semisynthetic process starting from podophyllotoxin. One common method involves the direct condensation of 4’-demethyl epipodophyllotoxin with 2,3-di-O-dichloroacetyl-(4,6-O-ethylidene)-β-D-glucopyranose in the presence of trimethylsilyl trifluoromethane sulfonate (TMSOTf) to yield 4’-demethylepipodophyllotoxin-4-(2,3-di-O-dichloroacetyl-4,6-0-ethylidene)-β-D-glucopyranoside, which is then converted to this compound . This method provides enhanced yields, reduced reaction times, and more favorable isolation procedures compared to existing techniques .
Analyse Chemischer Reaktionen
Etoposide unterliegt verschiedenen Arten von chemischen Reaktionen, darunter:
Reduktion: Reduktionsreaktionen können this compound in seine Hydrochinonform umwandeln.
Substitution: this compound kann Substitutionsreaktionen eingehen, insbesondere an der Glucopyranosid-Einheit.
Häufige Reagenzien und Bedingungen, die in diesen Reaktionen verwendet werden, umfassen Oxidationsmittel wie Wasserstoffperoxid und Reduktionsmittel wie Natriumborhydrid. Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, umfassen die O-Chinon- und Hydrochinon-Derivate von this compound .
Vergleich Mit ähnlichen Verbindungen
Etoposide ähnelt anderen Podophyllotoxin-Derivaten, wie z. B. Teniposid und Podophyllotoxin selbst. This compound ist einzigartig in seiner spezifischen Hemmung der Topoisomerase II und seiner Verwendung in einer breiten Palette von Krebsbehandlungen .
Podophyllotoxin: Der Naturstoff, von dem this compound abgeleitet ist, wird hauptsächlich wegen seiner antiviralen und antimitotischen Eigenschaften eingesetzt.
Die Einzigartigkeit von this compound liegt in seiner semisynthetischen Natur, seiner verbesserten Stabilität und seinem breiten Spektrum an Antikrebsaktivität .
Eigenschaften
CAS-Nummer |
33419-42-0 |
|---|---|
Molekularformel |
C29H32O13 |
Molekulargewicht |
588.6 g/mol |
IUPAC-Name |
(5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7S,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one |
InChI |
InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25+,26-,27-,29+/m1/s1 |
InChI-Schlüssel |
VJJPUSNTGOMMGY-QBUITQBFSA-N |
Verunreinigungen |
The following impurities are limited by the requirements of The British Pharmacopoeia: 4'-carbenzoxy ethylidene lignan P, picroethylidene lignan P, alpha-ethylidene lignan P, lignan P and 4'-demethylepipodophyllotoxin. |
SMILES |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
Isomerische SMILES |
C[C@@H]1OC[C@@H]2[C@@H](O1)[C@@H]([C@@H]([C@@H](O2)O[C@H]3[C@H]4COC(=O)[C@@H]4[C@@H](C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
Kanonische SMILES |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
Aussehen |
White to off-white solid powder |
Color/Form |
Crystals from methanol |
melting_point |
236-251 °C |
| 33419-42-0 | |
Physikalische Beschreibung |
Solid |
Piktogramme |
Irritant; Health Hazard |
Reinheit |
>98% (or refer to the Certificate of Analysis) |
Haltbarkeit |
>2 years if stored properly |
Löslichkeit |
Very soluble in methanol, chloroform; slightly soluble in ethanol, sparingly soluble in water. Sol in alc: approx 0.76 mg/ml Water solubility: approx 0.08 mg/mL |
Lagerung |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Synonyme |
alpha-D-Glucopyranosyl Isomer Etoposide Celltop Demethyl Epipodophyllotoxin Ethylidine Glucoside Eposide Eposin Eto GRY Eto-GRY Etomedac Etopos Etoposide Etoposide Pierre Fabre Etoposide Teva Etoposide, (5a alpha)-Isomer Etoposide, (5a alpha,9 alpha)-Isomer Etoposide, (5S)-Isomer Etoposide, alpha D Glucopyranosyl Isomer Etoposide, alpha-D-Glucopyranosyl Isomer Etoposido Ferrer Farma Exitop Lastet NSC 141540 NSC-141540 NSC141540 Onkoposid Riboposid Teva, Etoposide Toposar Vépéside Sandoz Vépéside-Sandoz Vepesid VP 16 VP 16 213 VP 16-213 VP 16213 VP-16 VP16 |
Dampfdruck |
5.4X10-23 mm Hg at 25 °C /Estimated/ |
Herkunft des Produkts |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary molecular target of Etoposide?
A1: this compound primarily targets DNA Topoisomerase II (Topo II), an enzyme essential for DNA replication and repair. [, ] It inhibits Topo II by trapping the enzyme in a complex with cleaved DNA, ultimately leading to DNA damage and cell death. []
Q2: How does this compound-induced DNA damage lead to cell death?
A2: this compound-induced DNA damage triggers a series of downstream events, including activation of p53, a tumor suppressor protein. [] p53 can initiate cell cycle arrest, giving the cell time to repair the damage, or, if the damage is too extensive, it can trigger apoptosis (programmed cell death). [, ]
Q3: What is the molecular formula and weight of this compound?
A3: While this specific information is not provided in the research excerpts, this compound's molecular formula is C29H32O13 and it has a molecular weight of 588.56 g/mol. This information can be readily found in publicly available chemical databases.
Q4: How does this compound perform in liposomal formulations for pulmonary delivery?
A5: Research indicates that this compound can be successfully incorporated into liposomes for pulmonary delivery. Freeze-dried liposomal formulations of this compound, using trehalose as a cryoprotectant, demonstrated good stability in terms of particle size and drug content for up to six months when stored at both ambient and refrigerated temperatures. []
Q5: What is the role of P-glycoprotein (P-gp) in the pharmacokinetics of this compound?
A6: P-glycoprotein (P-gp), encoded by the ABCB1 gene, plays a significant role in the absorption, distribution, and excretion of this compound. [, ] It acts as a transport protein, limiting the oral uptake of this compound and mediating its excretion across the gut wall. []
Q6: How does the ABCB1 (C1236T) polymorphism affect this compound's pharmacokinetics?
A7: The ABCB1 (C1236T) polymorphism has been shown to affect the transport activity of P-glycoprotein. Research using recombinant Caco-2 cell lines, expressing either the wild-type or variant P-gp, revealed that the variant P-gp transports this compound to a greater extent compared to the wild-type protein. [] This suggests that individuals with the ABCB1 (C1236T) polymorphism might experience altered this compound pharmacokinetics and potentially different therapeutic outcomes.
Q7: What is the bioavailability of oral this compound?
A8: The oral bioavailability of this compound is highly variable, ranging from 25% to 80% among cancer patients. [] This variability can be attributed, in part, to variations in transporter expression or activity, such as P-glycoprotein (P-gp), which influences the absorption and efflux of this compound. [, ]
Q8: What is the relationship between this compound exposure and neutropenia?
A9: Studies indicate a strong correlation between exposure to the free, pharmacologically active form of this compound and the risk of neutropenia, a significant decrease in neutrophils, a type of white blood cell. [] The higher the exposure to free this compound, the greater the risk of developing neutropenia.
Q9: What is the efficacy of oral this compound in treating metastatic breast cancer?
A10: A pooled analysis of twelve studies investigating the use of oral this compound in metastatic breast cancer revealed a moderate clinical effectiveness, with a pooled response rate of 18.5% and a clinical benefit rate of 45.8%. []
Q10: What are the known mechanisms of resistance to this compound?
A11: Resistance to this compound can arise through various mechanisms, including decreased expression of Topoisomerase II (Topo II), the primary target of this compound. [] Other mechanisms involve the multidrug-resistant phenotypes encoded by the mdr1 and MRP (multidrug resistance-associated protein) genes. []
Q11: What are the potential long-term effects of this compound treatment?
A13: this compound treatment has been associated with an increased risk of developing secondary acute myeloid leukemia (s-AML), a serious blood cancer. [] This risk appears to be higher when this compound is used in combination with cyclophosphamide. The latency period for developing s-AML after this compound treatment is typically 1-3 years, though longer periods have been reported. []
Q12: Have nanosuspensions been explored as a potential drug delivery system for this compound?
A14: Yes, research has investigated the use of this compound-loaded bovine serum albumin (BSA) nanosuspensions for parenteral delivery. [] This approach aims to improve the delivery of this compound, a poorly water-soluble drug, and potentially enhance its therapeutic efficacy while minimizing side effects.
Q13: What analytical techniques are commonly used to quantify this compound in biological samples?
A15: High-performance liquid chromatography (HPLC) is frequently employed to quantify this compound in biological samples, such as plasma. [, , ] Fluorescence detection is often used in conjunction with HPLC to enhance sensitivity. []
Q14: How do transporters like ABCC2 and ABCC3 influence this compound pharmacokinetics?
A16: ABCC2, also known as MRP2, plays a crucial role in the hepatobiliary excretion of this compound. [] ABCC3 (MRP3) contributes to the elimination of this compound glucuronide, a metabolite of this compound, from the liver into the bloodstream, which is subsequently eliminated in urine. []
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![2-methyl-N-[2-methyl-4-[(2-methylphenyl)diazenyl]phenyl]pyrazole-3-carboxamide](/img/structure/B1684373.png)
![2-[4,5-Bis[[bis(pyridin-2-ylmethyl)azaniumyl]methyl]-2,7-dichloro-3-oxido-6-oxoxanthen-9-yl]benzoate](/img/structure/B1684389.png)
![(5E)-5-[2-[(1E,3E)-5-hydroxy-5-[1-(3-phenylprop-2-ynyl)cyclobutyl]penta-1,3-dienyl]cyclohexylidene]pentanoic acid](/img/structure/B1684394.png)
![butyl 1-[(E,1R,4R)-4-[(1R,3aS,4E,7aR)-4-[(2Z)-2-[(3S,5R)-3,5-dihydroxy-2-methylidenecyclohexylidene]ethylidene]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-1-yl]-1-hydroxypent-2-enyl]cyclopropane-1-carboxylate](/img/structure/B1684395.png)
