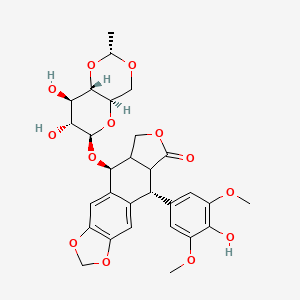
エトポシド
概要
説明
作用機序
科学的研究の応用
エトポシドは、以下のものを含む、幅広い科学研究における応用範囲を持っています。
準備方法
エトポシドは、ポドフィロトキシンを出発物質として、半合成プロセスによって合成されます。 一般的な方法の1つは、4'-脱メチルエピポドフィロトキシンと2,3-ジ-O-ジクロロアセチル-(4,6-O-エチリデン)-β-D-グルコピラノースを、トリメチルシリルトリフルオロメタンスルホネート(TMSOTf)の存在下で直接縮合させて、4'-脱メチルエピポドフィロトキシン-4-(2,3-ジ-O-ジクロロアセチル-4,6-O-エチリデン)-β-D-グルコピラノシドを得て、これをエトポシドに変換する方法です . この方法は、既存の手法と比較して、収率が向上し、反応時間が短縮され、分離操作が容易になるという利点があります .
化学反応の分析
エトポシドは、以下のものを含む、いくつかのタイプの化学反応を起こします。
酸化: エトポシドは、酸化されてO-キノン誘導体となり、これがDNAに対する活性に重要な役割を果たします.
還元: 還元反応により、エトポシドはヒドロキノン型に変換されます。
置換: エトポシドは、特にグルコピラノシド部分で、置換反応を起こす可能性があります。
これらの反応で使用される一般的な試薬および条件には、過酸化水素などの酸化剤と、水素化ホウ素ナトリウムなどの還元剤が含まれます。 これらの反応から生成される主要な生成物には、エトポシドのO-キノン誘導体とヒドロキノン誘導体が含まれます .
類似化合物との比較
エトポシドは、テニポシドやポドフィロトキシンなど、他のポドフィロトキシン誘導体と類似しています。 エトポシドは、トポイソメラーゼIIの特異的な阻害と、幅広い癌治療への利用において独自性を持ちます .
特性
CAS番号 |
33419-42-0 |
|---|---|
分子式 |
C29H32O13 |
分子量 |
588.6 g/mol |
IUPAC名 |
(5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7S,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one |
InChI |
InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25+,26-,27-,29+/m1/s1 |
InChIキー |
VJJPUSNTGOMMGY-QBUITQBFSA-N |
不純物 |
The following impurities are limited by the requirements of The British Pharmacopoeia: 4'-carbenzoxy ethylidene lignan P, picroethylidene lignan P, alpha-ethylidene lignan P, lignan P and 4'-demethylepipodophyllotoxin. |
SMILES |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
異性体SMILES |
C[C@@H]1OC[C@@H]2[C@@H](O1)[C@@H]([C@@H]([C@@H](O2)O[C@H]3[C@H]4COC(=O)[C@@H]4[C@@H](C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
正規SMILES |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
外観 |
White to off-white solid powder |
Color/Form |
Crystals from methanol |
melting_point |
236-251 °C |
| 33419-42-0 | |
物理的記述 |
Solid |
ピクトグラム |
Irritant; Health Hazard |
純度 |
>98% (or refer to the Certificate of Analysis) |
賞味期限 |
>2 years if stored properly |
溶解性 |
Very soluble in methanol, chloroform; slightly soluble in ethanol, sparingly soluble in water. Sol in alc: approx 0.76 mg/ml Water solubility: approx 0.08 mg/mL |
保存方法 |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
同義語 |
alpha-D-Glucopyranosyl Isomer Etoposide Celltop Demethyl Epipodophyllotoxin Ethylidine Glucoside Eposide Eposin Eto GRY Eto-GRY Etomedac Etopos Etoposide Etoposide Pierre Fabre Etoposide Teva Etoposide, (5a alpha)-Isomer Etoposide, (5a alpha,9 alpha)-Isomer Etoposide, (5S)-Isomer Etoposide, alpha D Glucopyranosyl Isomer Etoposide, alpha-D-Glucopyranosyl Isomer Etoposido Ferrer Farma Exitop Lastet NSC 141540 NSC-141540 NSC141540 Onkoposid Riboposid Teva, Etoposide Toposar Vépéside Sandoz Vépéside-Sandoz Vepesid VP 16 VP 16 213 VP 16-213 VP 16213 VP-16 VP16 |
蒸気圧 |
5.4X10-23 mm Hg at 25 °C /Estimated/ |
製品の起源 |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary molecular target of Etoposide?
A1: Etoposide primarily targets DNA Topoisomerase II (Topo II), an enzyme essential for DNA replication and repair. [, ] It inhibits Topo II by trapping the enzyme in a complex with cleaved DNA, ultimately leading to DNA damage and cell death. []
Q2: How does Etoposide-induced DNA damage lead to cell death?
A2: Etoposide-induced DNA damage triggers a series of downstream events, including activation of p53, a tumor suppressor protein. [] p53 can initiate cell cycle arrest, giving the cell time to repair the damage, or, if the damage is too extensive, it can trigger apoptosis (programmed cell death). [, ]
Q3: What is the molecular formula and weight of Etoposide?
A3: While this specific information is not provided in the research excerpts, Etoposide's molecular formula is C29H32O13 and it has a molecular weight of 588.56 g/mol. This information can be readily found in publicly available chemical databases.
Q4: How does Etoposide perform in liposomal formulations for pulmonary delivery?
A5: Research indicates that Etoposide can be successfully incorporated into liposomes for pulmonary delivery. Freeze-dried liposomal formulations of Etoposide, using trehalose as a cryoprotectant, demonstrated good stability in terms of particle size and drug content for up to six months when stored at both ambient and refrigerated temperatures. []
Q5: What is the role of P-glycoprotein (P-gp) in the pharmacokinetics of Etoposide?
A6: P-glycoprotein (P-gp), encoded by the ABCB1 gene, plays a significant role in the absorption, distribution, and excretion of Etoposide. [, ] It acts as a transport protein, limiting the oral uptake of Etoposide and mediating its excretion across the gut wall. []
Q6: How does the ABCB1 (C1236T) polymorphism affect Etoposide's pharmacokinetics?
A7: The ABCB1 (C1236T) polymorphism has been shown to affect the transport activity of P-glycoprotein. Research using recombinant Caco-2 cell lines, expressing either the wild-type or variant P-gp, revealed that the variant P-gp transports Etoposide to a greater extent compared to the wild-type protein. [] This suggests that individuals with the ABCB1 (C1236T) polymorphism might experience altered Etoposide pharmacokinetics and potentially different therapeutic outcomes.
Q7: What is the bioavailability of oral Etoposide?
A8: The oral bioavailability of Etoposide is highly variable, ranging from 25% to 80% among cancer patients. [] This variability can be attributed, in part, to variations in transporter expression or activity, such as P-glycoprotein (P-gp), which influences the absorption and efflux of Etoposide. [, ]
Q8: What is the relationship between Etoposide exposure and neutropenia?
A9: Studies indicate a strong correlation between exposure to the free, pharmacologically active form of Etoposide and the risk of neutropenia, a significant decrease in neutrophils, a type of white blood cell. [] The higher the exposure to free Etoposide, the greater the risk of developing neutropenia.
Q9: What is the efficacy of oral Etoposide in treating metastatic breast cancer?
A10: A pooled analysis of twelve studies investigating the use of oral Etoposide in metastatic breast cancer revealed a moderate clinical effectiveness, with a pooled response rate of 18.5% and a clinical benefit rate of 45.8%. []
Q10: What are the known mechanisms of resistance to Etoposide?
A11: Resistance to Etoposide can arise through various mechanisms, including decreased expression of Topoisomerase II (Topo II), the primary target of Etoposide. [] Other mechanisms involve the multidrug-resistant phenotypes encoded by the mdr1 and MRP (multidrug resistance-associated protein) genes. []
Q11: What are the potential long-term effects of Etoposide treatment?
A13: Etoposide treatment has been associated with an increased risk of developing secondary acute myeloid leukemia (s-AML), a serious blood cancer. [] This risk appears to be higher when Etoposide is used in combination with cyclophosphamide. The latency period for developing s-AML after Etoposide treatment is typically 1-3 years, though longer periods have been reported. []
Q12: Have nanosuspensions been explored as a potential drug delivery system for Etoposide?
A14: Yes, research has investigated the use of Etoposide-loaded bovine serum albumin (BSA) nanosuspensions for parenteral delivery. [] This approach aims to improve the delivery of Etoposide, a poorly water-soluble drug, and potentially enhance its therapeutic efficacy while minimizing side effects.
Q13: What analytical techniques are commonly used to quantify Etoposide in biological samples?
A15: High-performance liquid chromatography (HPLC) is frequently employed to quantify Etoposide in biological samples, such as plasma. [, , ] Fluorescence detection is often used in conjunction with HPLC to enhance sensitivity. []
Q14: How do transporters like ABCC2 and ABCC3 influence Etoposide pharmacokinetics?
A16: ABCC2, also known as MRP2, plays a crucial role in the hepatobiliary excretion of Etoposide. [] ABCC3 (MRP3) contributes to the elimination of Etoposide glucuronide, a metabolite of Etoposide, from the liver into the bloodstream, which is subsequently eliminated in urine. []
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![2-methyl-N-[2-methyl-4-[(2-methylphenyl)diazenyl]phenyl]pyrazole-3-carboxamide](/img/structure/B1684373.png)
![2-[4,5-Bis[[bis(pyridin-2-ylmethyl)azaniumyl]methyl]-2,7-dichloro-3-oxido-6-oxoxanthen-9-yl]benzoate](/img/structure/B1684389.png)
![(Z)-7-[(1R,2R,3R,5R)-5-chloro-2-[(E,3S)-3-cyclohexyl-3-hydroxyprop-1-enyl]-3-hydroxycyclopentyl]hept-5-enoic acid](/img/structure/B1684392.png)
![(5E)-5-[2-[(1E,3E)-5-hydroxy-5-[1-(3-phenylprop-2-ynyl)cyclobutyl]penta-1,3-dienyl]cyclohexylidene]pentanoic acid](/img/structure/B1684394.png)
