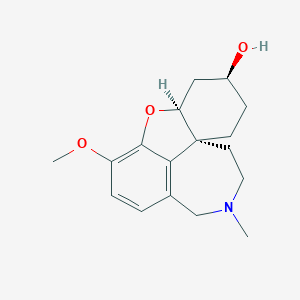
Lycoramine
Übersicht
Beschreibung
Lycoramin ist ein natürlich vorkommendes Alkaloid, das in den Zwiebeln von Pflanzen der Familie der Amaryllidaceae vorkommt, z. B. in Lycoris radiata. Es ist strukturell mit anderen Alkaloiden wie Galanthamin und Lycorin verwandt. Lycoramin hat aufgrund seiner potenziellen therapeutischen Anwendungen, insbesondere bei der Behandlung neurodegenerativer Erkrankungen wie der Alzheimer-Krankheit, Interesse geweckt .
Herstellungsmethoden
Synthetische Wege und Reaktionsbedingungen
Die Synthese von Lycoramin umfasst mehrere Schritte, darunter die Rhodium(I)-katalysierte [(3 + 2) + 1]-Cycloaddition von 1-En-Vinylcyclopropan und Kohlenmonoxid, um den cis-Hydrodibenzofuran-Kern zu erzeugen . Diese Methode ist effizient und wurde in der formalen Synthese von Lycoramin eingesetzt. Ein weiterer Ansatz beinhaltet die katalytische asymmetrische Hydrierung und die intramolekulare reduktive Heck-Cyclisierung .
Industrielle Produktionsmethoden
Die industrielle Produktion von Lycoramin ist nicht umfassend dokumentiert. Die oben genannten Synthesemethoden können für industrielle Anwendungen im großen Maßstab eingesetzt werden, sofern die Reaktionsbedingungen für die großtechnische Produktion optimiert werden.
Wirkmechanismus
Target of Action
Lycoramine, a natural compound extracted from Lycoris radiata, primarily targets acetylcholinesterase (AChE) . AChE is an enzyme involved in the rapid hydrolysis of the neurotransmitter acetylcholine, a critical component in the functioning of the nervous system .
Mode of Action
This compound acts as a potent inhibitor of AChE . By inhibiting AChE, this compound increases the concentration of acetylcholine in the synaptic cleft, enhancing cholinergic transmission. This action can have various effects depending on the specific neural pathways involved .
Biochemical Pathways
The biochemical pathways affected by this compound are primarily related to the cholinergic system due to its inhibition of AChE . Additionally, proteomics analysis has shown that this compound can cause statistically significant protein perturbations in the cortex, hippocampus, and cerebellum sections . These perturbations may affect various biochemical pathways, potentially contributing to the reversal of cognitive decline observed in Alzheimer’s disease models .
Pharmacokinetics
These properties can significantly impact the bioavailability of this compound, but more research is needed to provide a detailed understanding .
Result of Action
The primary molecular effect of this compound is the inhibition of AChE, leading to increased acetylcholine levels . At the cellular level, this can enhance cholinergic transmission, affecting various neural pathways. In Alzheimer’s disease models, this compound has been shown to reverse cognitive decline and clear Aβ plaques . These effects suggest that this compound may have therapeutic potential for neurodegenerative diseases .
Biochemische Analyse
Biochemical Properties
Lycoramine interacts with various enzymes and proteins in biochemical reactions. It is a potent inhibitor of acetylcholinesterase (AChE), an enzyme that breaks down acetylcholine, a key neurotransmitter . This interaction can influence various biochemical processes, particularly those related to nerve signal transmission.
Cellular Effects
This compound has been shown to have significant effects on various types of cells and cellular processes. For instance, it has demonstrated neuroprotective effects against CoCl2-induced SH-SY5Y cell injury . It also influences cell function by impacting cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
This compound exerts its effects at the molecular level through various mechanisms. As an AChE inhibitor, it binds to the active site of the enzyme, preventing the breakdown of acetylcholine and thereby enhancing cholinergic transmission . This binding interaction can lead to changes in gene expression and cellular metabolism, contributing to its various pharmacological effects .
Temporal Effects in Laboratory Settings
The effects of this compound can change over time in laboratory settings. For instance, the alkaloid content of this compound in Lycoris chinensis was found to increase with the age of the seedlings . This suggests that the product’s stability, degradation, and long-term effects on cellular function may vary depending on the duration of exposure and the age of the biological material.
Dosage Effects in Animal Models
The effects of this compound can vary with different dosages in animal models. In a study evaluating the therapeutic effects of this compound on Alzheimer’s disease in a mouse model, this compound showed therapeutic potential to halt and reverse cognitive decline at the late stages of disease progression .
Metabolic Pathways
This compound is involved in various metabolic pathways. It is part of the Amaryllidaceae alkaloids metabolism in Lycoris species . The alkaloid interacts with various enzymes and cofactors in these pathways, potentially affecting metabolic flux or metabolite levels .
Subcellular Localization
Given its interactions with enzymes such as AChE, which are located in the synaptic cleft in neurons, it is likely that this compound can localize to these areas within the cell .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions
The synthesis of lycoramine involves several steps, including the use of Rhodium(I)-catalyzed [(3 + 2) + 1] cycloaddition of 1-ene vinylcyclopropane and carbon monoxide to construct the cis-hydrodibenzofuran nucleus . This method is efficient and has been applied in the formal synthesis of this compound. Another approach involves catalytic asymmetric hydrogenation and intramolecular reductive Heck cyclization .
Industrial Production Methods
Industrial production of this compound is not extensively documented. the synthesis methods mentioned above can be scaled up for industrial applications, provided the reaction conditions are optimized for large-scale production.
Analyse Chemischer Reaktionen
Arten von Reaktionen
Lycoramin durchläuft verschiedene chemische Reaktionen, darunter:
Oxidation: Lycoramin kann oxidiert werden, um verschiedene Derivate zu bilden.
Reduktion: Katalytische asymmetrische Hydrierung wird bei seiner Synthese verwendet.
Substitution: Lycoramin kann Substitutionsreaktionen eingehen, um verschiedene Analoga zu bilden.
Häufige Reagenzien und Bedingungen
Oxidation: Es können gängige Oxidationsmittel wie Kaliumpermanganat oder Chromtrioxid verwendet werden.
Reduktion: Ruthenium- oder Palladiumkatalysatoren werden üblicherweise in Hydrierungsreaktionen verwendet.
Substitution: Verschiedene Nucleophile können unter geeigneten Bedingungen verwendet werden, um eine Substitution zu erreichen.
Hauptprodukte, die gebildet werden
Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, umfassen verschiedene Lycoramin-Derivate, die unterschiedliche pharmakologische Eigenschaften aufweisen können.
Wissenschaftliche Forschungsanwendungen
Lycoramin wurde wegen seiner potenziellen therapeutischen Wirkungen, insbesondere bei der Behandlung der Alzheimer-Krankheit, untersucht. Es hat sich gezeigt, dass es den kognitiven Verfall umkehrt und Amyloid-beta-Plaques in Mausmodellen beseitigt . Darüber hinaus wurde Lycoramin wegen seiner Acetylcholinesterase-Hemmeigenschaften untersucht, die für die Behandlung neurodegenerativer Erkrankungen relevant sind .
Wirkmechanismus
Lycoramin entfaltet seine Wirkung hauptsächlich durch die Hemmung der Acetylcholinesterase, eines Enzyms, das für den Abbau von Acetylcholin im Gehirn verantwortlich ist . Durch die Hemmung dieses Enzyms erhöht Lycoramin den Acetylcholinspiegel und verbessert so die kognitive Funktion. Darüber hinaus wurde gezeigt, dass Lycoramin mit verschiedenen molekularen Signalwegen interagiert, die an der Beseitigung von Amyloid-beta-Plaques beteiligt sind, die charakteristisch für die Alzheimer-Krankheit sind .
Vergleich Mit ähnlichen Verbindungen
Ähnliche Verbindungen
Galanthamin: Ein weiteres Alkaloid aus der Familie der Amaryllidaceae, das zur Behandlung der Alzheimer-Krankheit eingesetzt wird.
Crinin: Ein weiteres verwandtes Alkaloid mit potenziellen therapeutischen Anwendungen.
Einzigartigkeit von Lycoramin
Lycoramin ist einzigartig aufgrund seiner spezifischen molekularen Struktur, die es ihm ermöglicht, die Acetylcholinesterase effektiv zu hemmen und mit Signalwegen zu interagieren, die am Neuroprotektor beteiligt sind. Sein Potenzial, den kognitiven Verfall umzukehren und Amyloid-beta-Plaques zu beseitigen, macht es zu einem vielversprechenden Kandidaten für weitere Forschungsarbeiten zur Behandlung der Alzheimer-Krankheit .
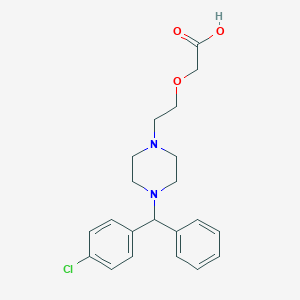
Eigenschaften
IUPAC Name |
(1R,12S,14S)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9-trien-14-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H23NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-4,12,14,19H,5-10H2,1-2H3/t12-,14-,17-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GJRMHIXYLGOZSE-JDFRZJQESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1CCC23CCC(CC2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN1CC[C@@]23CC[C@@H](C[C@@H]2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H23NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID30175371 | |
| Record name | Lycoramine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID30175371 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
289.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
21133-52-8 | |
| Record name | Lycoramine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0021133528 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Lycoramine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID30175371 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 21133-52-8 | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LYCORAMINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/TAG8LU84K2 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of lycoramine?
A1: this compound primarily acts as an acetylcholinesterase (AChE) inhibitor. [, , , ] This means it blocks the action of the enzyme responsible for breaking down the neurotransmitter acetylcholine, leading to increased levels of acetylcholine in the synaptic cleft.
Q2: How does this compound's AChE inhibitory activity compare to other known inhibitors?
A2: Studies show that this compound is a more potent AChE inhibitor than physostigmine but less potent than its close structural analog, galanthamine. [, , ] The potency of this compound derivatives can vary depending on the specific structural modifications. [, , ]
Q3: Beyond AChE inhibition, does this compound interact with other targets?
A3: Research suggests that this compound may also interact with nicotinic acetylcholine receptors, although the specific mechanisms and implications require further investigation. []
Q4: What are the downstream effects of this compound's AChE inhibition?
A4: By inhibiting AChE, this compound enhances cholinergic neurotransmission. This has been linked to several observed effects, including:
- Increased smooth muscle activity: Observed as increased tonus and peristalsis of the intestines in animal models. [, ]
- Pupillary constriction: this compound can cause miosis, which can be counteracted by atropine, suggesting an interaction with muscarinic acetylcholine receptors. [, ]
- Potentiation of muscle contractions: this compound can enhance the response of skeletal muscles to nerve stimulation, both directly and by potentiating the effects of acetylcholine. [, , ] This effect is dependent on dosage and stimulation frequency.
- Cognitive enhancement: Studies in a mouse model of Alzheimer's disease showed that this compound administration improved cognitive performance in behavioral tests, suggesting a potential role in mitigating cognitive decline. []
Q5: What is the molecular formula and weight of this compound?
A5: The molecular formula of this compound is C17H21NO4, and its molecular weight is 303.35 g/mol. [, , ]
Q6: What spectroscopic data is available for characterizing this compound?
A6: Spectroscopic techniques, including ultraviolet (UV), infrared (IR), nuclear magnetic resonance (NMR), and mass spectrometry (MS), have been extensively used to characterize this compound. [, , , , ]
- UV Spectroscopy: this compound exhibits characteristic UV absorption maxima, which can be used for its identification and quantification. []
- IR Spectroscopy: The IR spectrum of this compound reveals the presence of specific functional groups, such as hydroxyl (-OH) and aromatic rings. [, ]
- NMR Spectroscopy: Detailed 1H and 13C NMR data provide insights into the structure and stereochemistry of this compound and its derivatives. [, , , ]
- Mass Spectrometry: MS techniques are used to determine the molecular weight of this compound and analyze its fragmentation patterns, aiding in its structural elucidation. [, ]
Q7: What is the typical route of administration for this compound in research settings?
A7: this compound has been administered intravenously, intra-arterially, and subcutaneously in animal studies. [, , , ]
Q8: Has this compound been evaluated in clinical trials for any specific conditions?
A8: While this compound has shown promising results in preclinical studies, particularly in animal models of Alzheimer's disease, it has not yet progressed to clinical trials. [, ]
Q9: What is the toxicity profile of this compound?
A9: this compound's toxicity profile has been studied in mice, where its LD50 (the dose at which 50% of the animals die) was found to be 16.65 mg/kg after subcutaneous injection. [] This is considerably higher than the LD50 of galanthamine (0.958 mg/kg) and neostigmine (0.174 mg/kg), indicating a potentially wider therapeutic window. [, ]
Q10: What are the typical toxic symptoms observed with this compound overdose?
A10: Common toxic symptoms in animal models include salivation, muscle twitching, convulsions, and respiratory paralysis, which is the usual cause of death. [, ]
Q11: Which plant species are considered good sources of this compound?
A11: Several species within the Amaryllidaceae family are known to contain this compound, including:
Q12: What extraction methods are commonly employed for isolating this compound?
A12: Various extraction methods have been explored for isolating this compound from plant materials, including:
- Soxhlet extraction: A traditional method using organic solvents. []
- Microwave-assisted extraction (MAE): This technique utilizes microwave energy to heat the solvent and plant material, enhancing extraction efficiency and reducing extraction time. [, ]
- Post-microwave-irradiated reflux extraction (PMIRE): This method combines microwave irradiation with conventional reflux extraction, potentially improving extraction yields. []
Q13: How do structural modifications of this compound influence its AChE inhibitory activity?
A13: Several studies have investigated the structure-activity relationship of this compound and its derivatives:
- Quaternization of the nitrogen atom: Converting this compound to its quaternary ammonium salt significantly reduces its ability to cross the blood-brain barrier, limiting its central nervous system effects. []
- Introduction of carbamate groups: The introduction of dimethylcarbamate groups to the this compound structure has been shown to increase its AChE inhibitory activity compared to the parent compound. [, , ]
- Modifications to the aromatic ring: Changes in the substitution pattern on the aromatic ring can affect both the potency and selectivity of this compound derivatives toward AChE. []
Q14: What analytical techniques are commonly employed for detecting and quantifying this compound?
A14: Several analytical methods have been developed for the determination of this compound in plant extracts and biological samples, including:
- High-performance liquid chromatography (HPLC): Often coupled with UV or fluorescence detection, HPLC provides a sensitive and selective method for quantifying this compound. [, , , , ]
- Gas chromatography-mass spectrometry (GC-MS): This technique offers high sensitivity and selectivity for the identification and quantification of this compound, often used in combination with microwave-assisted extraction. [, ]
- Flow-injection chemiluminescence (FI-CL): This method utilizes the chemiluminescence reaction of luminol with potassium ferricyanide, which is inhibited by this compound, allowing for its sensitive detection. []
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![1,4-Bis[(4-chlorophenyl)phenylmethyl]piperazine dihydrochloride](/img/structure/B192774.png)