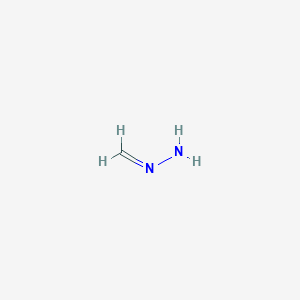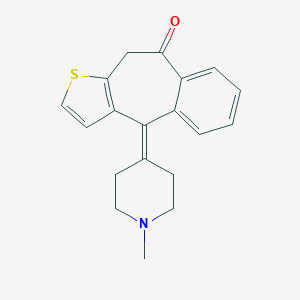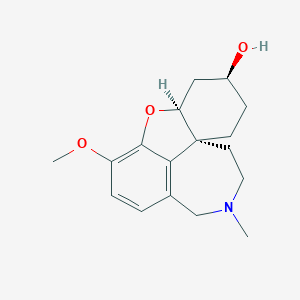
リコラミン
概要
説明
リコラミンは、ヒガンバナ科の植物(ヒガンバナなど)の球根に含まれる天然アルカロイドです。ガランタミンやリコリンなどの他のアルカロイドと構造的に関連しています。 リコラミンは、特にアルツハイマー病などの神経変性疾患の治療における潜在的な治療用途により注目を集めています .
科学的研究の応用
Lycoramine has been studied for its potential therapeutic effects, particularly in the treatment of Alzheimer’s disease. It has shown promise in reversing cognitive decline and clearing amyloid-beta plaques in mouse models . Additionally, lycoramine has been investigated for its acetylcholinesterase inhibition properties, which are relevant in the treatment of neurodegenerative diseases .
作用機序
リコラミンは、主に脳内のアセチルコリンの分解を担う酵素であるアセチルコリンエステラーゼの阻害を通じて作用を発揮します 。この酵素を阻害することにより、リコラミンはアセチルコリンのレベルを高め、認知機能を改善します。 さらに、リコラミンは、アルツハイマー病の特徴であるアミロイドβプラークのクリアランスに関与するさまざまな分子経路と相互作用することが示されています .
類似の化合物との比較
類似の化合物
ガランタミン: ヒガンバナ科の別のアルカロイドで、アルツハイマー病の治療に使用されています。
リコリン: リコラミンと構造的に類似しており、抗炎症作用や抗ウイルス作用など、さまざまな薬理学的効果を持っています.
クリニン: 潜在的な治療用途を持つ別の関連するアルカロイドです。
リコラミンの独自性
リコラミンは、アセチルコリンエステラーゼを効果的に阻害し、神経保護に関与する経路と相互作用することを可能にする特定の分子構造によって独特です。 認知機能の低下を回復させ、アミロイドβプラークをクリアする可能性は、アルツハイマー病の治療におけるさらなる研究のための有望な候補となっています .
生化学分析
Biochemical Properties
Lycoramine interacts with various enzymes and proteins in biochemical reactions. It is a potent inhibitor of acetylcholinesterase (AChE), an enzyme that breaks down acetylcholine, a key neurotransmitter . This interaction can influence various biochemical processes, particularly those related to nerve signal transmission.
Cellular Effects
Lycoramine has been shown to have significant effects on various types of cells and cellular processes. For instance, it has demonstrated neuroprotective effects against CoCl2-induced SH-SY5Y cell injury . It also influences cell function by impacting cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
Lycoramine exerts its effects at the molecular level through various mechanisms. As an AChE inhibitor, it binds to the active site of the enzyme, preventing the breakdown of acetylcholine and thereby enhancing cholinergic transmission . This binding interaction can lead to changes in gene expression and cellular metabolism, contributing to its various pharmacological effects .
Temporal Effects in Laboratory Settings
The effects of lycoramine can change over time in laboratory settings. For instance, the alkaloid content of Lycoramine in Lycoris chinensis was found to increase with the age of the seedlings . This suggests that the product’s stability, degradation, and long-term effects on cellular function may vary depending on the duration of exposure and the age of the biological material.
Dosage Effects in Animal Models
The effects of lycoramine can vary with different dosages in animal models. In a study evaluating the therapeutic effects of lycoramine on Alzheimer’s disease in a mouse model, lycoramine showed therapeutic potential to halt and reverse cognitive decline at the late stages of disease progression .
Metabolic Pathways
Lycoramine is involved in various metabolic pathways. It is part of the Amaryllidaceae alkaloids metabolism in Lycoris species . The alkaloid interacts with various enzymes and cofactors in these pathways, potentially affecting metabolic flux or metabolite levels .
Subcellular Localization
Given its interactions with enzymes such as AChE, which are located in the synaptic cleft in neurons, it is likely that lycoramine can localize to these areas within the cell .
準備方法
合成経路と反応条件
リコラミンの合成には、シス-ヒドロジベンゾフラン核を構築するためのロジウム(I)触媒による [(3 + 2) + 1] 環状付加反応など、いくつかのステップが含まれます 。この方法は効率的であり、リコラミンの正式合成に適用されています。 別の方法には、触媒的不斉水素化と分子内還元ヘック環化が含まれます .
工業生産方法
リコラミンの工業生産は広く文書化されていません。上記の合成方法は、反応条件が大型生産用に最適化されていれば、工業用途にスケールアップできます。
化学反応の分析
反応の種類
リコラミンは、次のようなさまざまな化学反応を起こします。
酸化: リコラミンは、酸化されてさまざまな誘導体を形成することができます。
還元: 触媒的不斉水素化はその合成に使用されます.
置換: リコラミンは、さまざまなアナログを形成するために置換反応を起こすことができます。
一般的な試薬と条件
酸化: 過マンガン酸カリウムや三酸化クロムなどの一般的な酸化剤を使用できます。
還元: ルテニウムまたはパラジウム触媒は、水素化反応で一般的に使用されます.
置換: 適切な条件下で、さまざまな求核剤を使用することで置換反応を実現できます。
生成される主な生成物
これらの反応から生成される主な生成物には、さまざまな薬理学的特性を持つさまざまなリコラミン誘導体が含まれます。
科学研究への応用
リコラミンは、特にアルツハイマー病の治療における潜在的な治療効果について研究されています。 マウスモデルにおいて、認知機能の低下を回復させ、アミロイドβプラークをクリアする有望な結果を示しています 。 さらに、リコラミンは、神経変性疾患の治療に関連するアセチルコリンエステラーゼ阻害特性について調査されています .
類似化合物との比較
Similar Compounds
Galanthamine: Another alkaloid from the Amaryllidaceae family, used in the treatment of Alzheimer’s disease.
Lycorine: Structurally similar to lycoramine and has various pharmacological effects, including anti-inflammatory and antiviral properties.
Crinine: Another related alkaloid with potential therapeutic applications.
Uniqueness of Lycoramine
Lycoramine is unique due to its specific molecular structure, which allows it to effectively inhibit acetylcholinesterase and interact with pathways involved in neuroprotection. Its potential to reverse cognitive decline and clear amyloid-beta plaques makes it a promising candidate for further research in the treatment of Alzheimer’s disease .
特性
IUPAC Name |
(1R,12S,14S)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9-trien-14-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H23NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-4,12,14,19H,5-10H2,1-2H3/t12-,14-,17-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GJRMHIXYLGOZSE-JDFRZJQESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1CCC23CCC(CC2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN1CC[C@@]23CC[C@@H](C[C@@H]2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H23NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID30175371 | |
| Record name | Lycoramine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID30175371 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
289.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
21133-52-8 | |
| Record name | Lycoramine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0021133528 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Lycoramine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID30175371 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 21133-52-8 | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LYCORAMINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/TAG8LU84K2 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of lycoramine?
A1: Lycoramine primarily acts as an acetylcholinesterase (AChE) inhibitor. [, , , ] This means it blocks the action of the enzyme responsible for breaking down the neurotransmitter acetylcholine, leading to increased levels of acetylcholine in the synaptic cleft.
Q2: How does lycoramine's AChE inhibitory activity compare to other known inhibitors?
A2: Studies show that lycoramine is a more potent AChE inhibitor than physostigmine but less potent than its close structural analog, galanthamine. [, , ] The potency of lycoramine derivatives can vary depending on the specific structural modifications. [, , ]
Q3: Beyond AChE inhibition, does lycoramine interact with other targets?
A3: Research suggests that lycoramine may also interact with nicotinic acetylcholine receptors, although the specific mechanisms and implications require further investigation. []
Q4: What are the downstream effects of lycoramine's AChE inhibition?
A4: By inhibiting AChE, lycoramine enhances cholinergic neurotransmission. This has been linked to several observed effects, including:
- Increased smooth muscle activity: Observed as increased tonus and peristalsis of the intestines in animal models. [, ]
- Pupillary constriction: Lycoramine can cause miosis, which can be counteracted by atropine, suggesting an interaction with muscarinic acetylcholine receptors. [, ]
- Potentiation of muscle contractions: Lycoramine can enhance the response of skeletal muscles to nerve stimulation, both directly and by potentiating the effects of acetylcholine. [, , ] This effect is dependent on dosage and stimulation frequency.
- Cognitive enhancement: Studies in a mouse model of Alzheimer's disease showed that lycoramine administration improved cognitive performance in behavioral tests, suggesting a potential role in mitigating cognitive decline. []
Q5: What is the molecular formula and weight of lycoramine?
A5: The molecular formula of lycoramine is C17H21NO4, and its molecular weight is 303.35 g/mol. [, , ]
Q6: What spectroscopic data is available for characterizing lycoramine?
A6: Spectroscopic techniques, including ultraviolet (UV), infrared (IR), nuclear magnetic resonance (NMR), and mass spectrometry (MS), have been extensively used to characterize lycoramine. [, , , , ]
- UV Spectroscopy: Lycoramine exhibits characteristic UV absorption maxima, which can be used for its identification and quantification. []
- IR Spectroscopy: The IR spectrum of lycoramine reveals the presence of specific functional groups, such as hydroxyl (-OH) and aromatic rings. [, ]
- NMR Spectroscopy: Detailed 1H and 13C NMR data provide insights into the structure and stereochemistry of lycoramine and its derivatives. [, , , ]
- Mass Spectrometry: MS techniques are used to determine the molecular weight of lycoramine and analyze its fragmentation patterns, aiding in its structural elucidation. [, ]
Q7: What is the typical route of administration for lycoramine in research settings?
A7: Lycoramine has been administered intravenously, intra-arterially, and subcutaneously in animal studies. [, , , ]
Q8: Has lycoramine been evaluated in clinical trials for any specific conditions?
A8: While lycoramine has shown promising results in preclinical studies, particularly in animal models of Alzheimer's disease, it has not yet progressed to clinical trials. [, ]
Q9: What is the toxicity profile of lycoramine?
A9: Lycoramine's toxicity profile has been studied in mice, where its LD50 (the dose at which 50% of the animals die) was found to be 16.65 mg/kg after subcutaneous injection. [] This is considerably higher than the LD50 of galanthamine (0.958 mg/kg) and neostigmine (0.174 mg/kg), indicating a potentially wider therapeutic window. [, ]
Q10: What are the typical toxic symptoms observed with lycoramine overdose?
A10: Common toxic symptoms in animal models include salivation, muscle twitching, convulsions, and respiratory paralysis, which is the usual cause of death. [, ]
Q11: Which plant species are considered good sources of lycoramine?
A11: Several species within the Amaryllidaceae family are known to contain lycoramine, including:
Q12: What extraction methods are commonly employed for isolating lycoramine?
A12: Various extraction methods have been explored for isolating lycoramine from plant materials, including:
- Soxhlet extraction: A traditional method using organic solvents. []
- Microwave-assisted extraction (MAE): This technique utilizes microwave energy to heat the solvent and plant material, enhancing extraction efficiency and reducing extraction time. [, ]
- Post-microwave-irradiated reflux extraction (PMIRE): This method combines microwave irradiation with conventional reflux extraction, potentially improving extraction yields. []
Q13: How do structural modifications of lycoramine influence its AChE inhibitory activity?
A13: Several studies have investigated the structure-activity relationship of lycoramine and its derivatives:
- Quaternization of the nitrogen atom: Converting lycoramine to its quaternary ammonium salt significantly reduces its ability to cross the blood-brain barrier, limiting its central nervous system effects. []
- Introduction of carbamate groups: The introduction of dimethylcarbamate groups to the lycoramine structure has been shown to increase its AChE inhibitory activity compared to the parent compound. [, , ]
- Modifications to the aromatic ring: Changes in the substitution pattern on the aromatic ring can affect both the potency and selectivity of lycoramine derivatives toward AChE. []
Q14: What analytical techniques are commonly employed for detecting and quantifying lycoramine?
A14: Several analytical methods have been developed for the determination of lycoramine in plant extracts and biological samples, including:
- High-performance liquid chromatography (HPLC): Often coupled with UV or fluorescence detection, HPLC provides a sensitive and selective method for quantifying lycoramine. [, , , , ]
- Gas chromatography-mass spectrometry (GC-MS): This technique offers high sensitivity and selectivity for the identification and quantification of lycoramine, often used in combination with microwave-assisted extraction. [, ]
- Flow-injection chemiluminescence (FI-CL): This method utilizes the chemiluminescence reaction of luminol with potassium ferricyanide, which is inhibited by lycoramine, allowing for its sensitive detection. []
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。