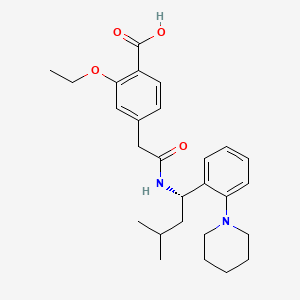
Repaglinida
Descripción general
Descripción
Pertenece a la clase de meglitinidas de secretagogos de insulina de acción corta, que actúan uniéndose a las células β del páncreas para estimular la liberación de insulina . La repaglinida es conocida por su inicio rápido y corta duración de acción, lo que la hace efectiva para controlar los niveles de glucosa en sangre posprandial .
Aplicaciones Científicas De Investigación
La repaglinida tiene varias aplicaciones de investigación científica, que incluyen:
Química: Utilizada como un compuesto modelo para estudiar la síntesis y modificación de fármacos antidiabéticos.
Biología: Estudiada por sus efectos sobre las células β pancreáticas y la secreción de insulina.
Medicina: Utilizada en ensayos clínicos para evaluar su eficacia y seguridad en el tratamiento de la diabetes tipo 2.
Industria: Empleada en el desarrollo de nuevas formulaciones de fármacos y sistemas de administración para mejorar la biodisponibilidad y los resultados terapéuticos
Mecanismo De Acción
La repaglinida ejerce sus efectos uniéndose a los canales de potasio sensibles al ATP en las células β del páncreas. Esta unión inhibe la salida de iones de potasio, lo que lleva a la despolarización de la membrana celular. La despolarización abre los canales de calcio dependientes de voltaje, permitiendo que los iones de calcio entren en la célula. La entrada de iones de calcio desencadena la exocitosis de los gránulos de insulina, lo que resulta en un aumento de la liberación de insulina . Este mecanismo es dependiente de la glucosa, lo que significa que la liberación de insulina solo se estimula en presencia de glucosa, lo que reduce el riesgo de hipoglucemia .
Análisis Bioquímico
Biochemical Properties
Repaglinide interacts with specific proteins in the body, particularly in the pancreas. It binds to ATP-sensitive potassium channels on the surface of pancreatic beta cells . This binding inhibits potassium efflux, leading to depolarization of the cell membrane and subsequent insulin release .
Cellular Effects
Repaglinide has a profound effect on pancreatic beta cells. By stimulating insulin release, it helps regulate blood glucose levels. It also influences cell signaling pathways related to insulin secretion .
Molecular Mechanism
The molecular mechanism of Repaglinide involves its binding to ATP-sensitive potassium channels on pancreatic beta cells . This binding inhibits the efflux of potassium ions, causing the cell to depolarize. This depolarization triggers the opening of calcium channels, leading to an influx of calcium ions, which then stimulate the release of insulin .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Repaglinide are observed over time. It has been found to have a sustained release, ensuring safety and improving the efficacy of the drug . The drug’s stability and degradation over time are factors that are considered in its formulation .
Dosage Effects in Animal Models
The effects of Repaglinide in animal models vary with dosage. Studies have shown that it effectively lowers blood glucose levels in a dose-dependent manner .
Metabolic Pathways
Repaglinide is involved in metabolic pathways related to glucose regulation. It interacts with enzymes and cofactors in these pathways, influencing metabolic flux and metabolite levels .
Transport and Distribution
Repaglinide is transported and distributed within cells and tissues via specific transporters . Its localization and accumulation within cells can influence its efficacy .
Subcellular Localization
The subcellular localization of Repaglinide is primarily at the cell membrane of pancreatic beta cells, where it interacts with ATP-sensitive potassium channels . This localization is crucial for its function in stimulating insulin release .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción
La repaglinida se puede sintetizar a través de un proceso de varios pasos que involucra varios intermediarios clave. Un método común involucra los siguientes pasos :
Esterificación: El ácido 3-hidroxifenilacético se esterifica para formar 3-hidroxifenilacetato de etilo.
Formilación: El éster se formila para producir 3-formil-4-hidroxifenilacetato de etilo.
Oxidación: El grupo formilo se oxida a un ácido carboxílico, produciendo 3-carboxi-4-hidroxifenilacetato de etilo.
Eterificación: El grupo hidroxilo se eterifica para formar 3-carboxi-4-etoxifenilacetato de etilo.
Hidrólisis selectiva: El éster se hidroliza selectivamente para producir ácido 3-carboxi-4-etoxifenilacético, un intermediario clave en la síntesis de this compound.
Métodos de producción industrial
La producción industrial de this compound implica la optimización de las condiciones de reacción para maximizar el rendimiento y la pureza. Esto incluye controlar la temperatura de reacción, el tiempo, el solvente y las relaciones de sustrato . El proceso está diseñado para ser escalable y ecológico, con mínimas impurezas.
Análisis De Reacciones Químicas
Tipos de reacciones
La repaglinida sufre varios tipos de reacciones químicas, que incluyen:
Oxidación: La this compound se puede oxidar para formar varios metabolitos.
Reducción: Las reacciones de reducción pueden modificar los grupos funcionales en la molécula de this compound.
Sustitución: Las reacciones de sustitución pueden ocurrir en varias posiciones en el anillo aromático y las cadenas laterales.
Reactivos y condiciones comunes
Los reactivos comunes utilizados en la síntesis y modificación de la this compound incluyen:
Agentes oxidantes: Como el permanganato de potasio y el peróxido de hidrógeno.
Agentes reductores: Como el borohidruro de sodio y el hidruro de litio y aluminio.
Reactivos de sustitución: Como halógenos y agentes alquilantes.
Productos principales
Los productos principales formados a partir de estas reacciones incluyen varios metabolitos y derivados de this compound, que se pueden caracterizar utilizando técnicas como espectroscopia FT-IR, RMN y UV-Vis .
Comparación Con Compuestos Similares
La repaglinida se compara a menudo con otros fármacos antidiabéticos, como:
Nateglinida: Otra meglitinida con un mecanismo de acción similar pero una duración de efecto más corta.
Sulfonilureas: Como la glibenclamida y la glimepirida, que también estimulan la liberación de insulina pero tienen una duración de acción más larga y un mayor riesgo de hipoglucemia.
Metformina: Una biguanida que reduce la producción hepática de glucosa y mejora la sensibilidad a la insulina, pero no estimula la liberación de insulina
La this compound es única en su inicio rápido y corta duración de acción, lo que la hace particularmente efectiva para controlar los niveles de glucosa en sangre posprandial sin causar hipoglucemia prolongada .
Propiedades
IUPAC Name |
2-ethoxy-4-[2-[[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H36N2O4/c1-4-33-25-17-20(12-13-22(25)27(31)32)18-26(30)28-23(16-19(2)3)21-10-6-7-11-24(21)29-14-8-5-9-15-29/h6-7,10-13,17,19,23H,4-5,8-9,14-16,18H2,1-3H3,(H,28,30)(H,31,32)/t23-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FAEKWTJYAYMJKF-QHCPKHFHSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC1=C(C=CC(=C1)CC(=O)NC(CC(C)C)C2=CC=CC=C2N3CCCCC3)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC1=C(C=CC(=C1)CC(=O)N[C@@H](CC(C)C)C2=CC=CC=C2N3CCCCC3)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H36N2O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3023552 | |
| Record name | Repaglinide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3023552 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
452.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Repaglinide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015048 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>67.9 [ug/mL] (The mean of the results at pH 7.4), 2.94e-03 g/L | |
| Record name | SID49648522 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Repaglinide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015048 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Repaglinide activity is dependent on the presence functioning β cells and glucose. In contrast to sulfonylurea insulin secretatogogues, repaglinide has no effect on insulin release in the absence of glucose. Rather, it potentiates the effect of extracellular glucose on ATP-sensitive potassium channel and has little effect on insulin levels between meals and overnight. As such, repaglinide is more effective at reducing postprandial blood glucose levels than fasting blood glucose levels and requires a longer duration of therapy (approximately one month) before decreases in fasting blood glucose are observed. The insulinotropic effects of repaglinide are highest at intermediate glucose levels (3 to 10 mmol/L) and it does not increase insulin release already stimulated by high glucose concentrations (greater than 15 mmol/L). Repaglinide appears to be selective for pancreatic β cells and does not appear to affect skeletal or cardiac muscle or thyroid tissue. | |
| Record name | Repaglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
135062-02-1 | |
| Record name | Repaglinide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=135062-02-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Repaglinide [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0135062021 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Repaglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Repaglinide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759893 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Repaglinide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3023552 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (+)-2-Ethoxy-alpha-(((S)-alpha-isobutyl-o-piperidinobenzyl)carbamoyl)-p-toluic acid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | REPAGLINIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/668Z8C33LU | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Repaglinide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015048 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
130-131 °C, 130 - 131 °C | |
| Record name | Repaglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Repaglinide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015048 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details






Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of repaglinide?
A: Repaglinide is a non-sulfonylurea insulin secretagogue. It works by binding to and blocking ATP-sensitive potassium (K-ATP) channels on the surface of pancreatic beta cells. [, , , , ] This blockage depolarizes the beta cells, leading to the opening of voltage-gated calcium channels. The influx of calcium ions triggers the release of insulin from the beta cells. [, , , ]
Q2: How does the action of repaglinide differ from sulfonylureas?
A: While both repaglinide and sulfonylureas stimulate insulin release by binding to the sulfonylurea receptor 1 (SUR1) subunit of the K-ATP channel, they do so at distinct binding sites. [] This difference translates to a faster onset and shorter duration of action for repaglinide compared to sulfonylureas. [, , , , , ]
Q3: What is the impact of repaglinide on insulin secretion patterns?
A: Repaglinide primarily amplifies the mass and amplitude of insulin secretory bursts without affecting the frequency of these bursts. [] This action enhances both early- and late-phase insulin secretion in response to hyperglycemia. []
Q4: What is the molecular formula and weight of repaglinide?
A: Repaglinide has the molecular formula C27H36N2O4 and a molecular weight of 452.58 g/mol. []
Q5: Is there any spectroscopic data available for repaglinide?
A: Yes, repaglinide shows maximum UV absorbance at a wavelength of 237 nm in both phosphate buffer (pH 7.4) and 0.1 N HCl solutions. [] Fourier-transform infrared spectroscopy (FT-IR) analysis reveals characteristic peaks for repaglinide. [, ]
Q6: How does co-administration of repaglinide with cytochrome P450 (CYP)3A4 inhibitors affect its pharmacokinetics?
A: Strong CYP3A4 inhibitors, like ketoconazole, can increase the area under the curve (AUC) and peak plasma concentration (Cmax) of repaglinide, although to a lesser extent than expected due to its metabolism by multiple CYP enzymes. [] This interaction may necessitate adjustments in repaglinide dosage and blood glucose monitoring. []
Q7: Does the co-administration of repaglinide with CYP3A4 inducers impact its effectiveness?
A: Rifampicin, a potent CYP3A4 inducer, can significantly decrease the AUC and Cmax of repaglinide. [, , ] This interaction can potentially reduce the glucose-lowering effects of repaglinide, requiring dose adjustments and close monitoring. []
Q8: How does trimethoprim affect the pharmacokinetics and pharmacodynamics of repaglinide?
A: Trimethoprim, a CYP2C8 inhibitor, can significantly increase the plasma concentrations of repaglinide by inhibiting its CYP2C8-mediated metabolism. [, ] This interaction may increase the risk of hypoglycemia, particularly at higher doses. [, ]
Q9: Does grapefruit juice consumption impact the pharmacokinetics of repaglinide?
A: Grapefruit juice, a known inhibitor of intestinal CYP3A4, can increase the bioavailability of repaglinide, potentially leading to higher plasma concentrations. [] This effect is more prominent at lower doses of repaglinide. []
Q10: What is the absorption profile of repaglinide?
A: Repaglinide is rapidly and completely absorbed from the gastrointestinal tract after oral administration. [, , ]
Q11: What is the time to peak plasma concentration (Tmax) for repaglinide?
A: Peak plasma levels (Cmax) are achieved within 1 hour (Tmax) of administration. [, ]
Q12: How is repaglinide metabolized and eliminated from the body?
A: Repaglinide is primarily metabolized in the liver by CYP enzymes, mainly CYP2C8 and CYP3A4, into inactive metabolites. [, , , ] These metabolites are subsequently excreted primarily in bile. [, ]
Q13: Does the presence of the CYP2C8*3 allele affect the pharmacokinetics of repaglinide?
A: Contrary to some previous studies, research indicates that the CYP2C8*3 allele does not significantly alter the pharmacokinetics of repaglinide at therapeutic doses. []
Q14: Does the SLCO1B1 gene, which encodes for the OATP1B1 transporter, influence repaglinide disposition?
A: While pitavastatin, an OATP1B1 inhibitor, can increase repaglinide Cmax in individuals with specific SLCO1B1 genotypes, it does not significantly affect its overall pharmacokinetics or pharmacodynamics. [, ]
Q15: What is the primary therapeutic use of repaglinide?
A: Repaglinide is primarily indicated for the treatment of type 2 diabetes mellitus, particularly in improving glycemic control. [, , , , , , ]
Q16: How does repaglinide compare to other antidiabetic agents in terms of efficacy?
A: In clinical trials, repaglinide demonstrated comparable efficacy to glibenclamide and gliclazide in improving glycemic control. [] It also showed superior efficacy to glipizide in maintaining glycemic control over a year. []
Q17: What is the role of repaglinide in combination therapy for type 2 diabetes?
A: Repaglinide is often used in combination with other antidiabetic agents, such as metformin or thiazolidinediones, to enhance glycemic control. [, , , , , , ]
Q18: Can repaglinide be used as monotherapy in the treatment of type 2 diabetes?
A: Yes, clinical trials have shown repaglinide monotherapy to be effective in improving glycemic control in patients with type 2 diabetes. []
Q19: What formulation strategies have been explored to improve the solubility and dissolution rate of repaglinide?
A: Solid dispersions of repaglinide with polymers like polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), mannitol, and urea have been investigated to enhance its solubility and dissolution rate. [, , , ]
Q20: Have any specific formulations, such as fast-dissolving tablets, been developed for repaglinide?
A: Yes, fast-dissolving tablets incorporating repaglinide solid dispersions and superdisintegrants have been developed to improve its bioavailability and potentially enhance its therapeutic efficacy. []
Q21: What is the rationale behind developing transdermal patches for repaglinide delivery?
A: Transdermal patches have been explored to achieve sustained release of repaglinide, improve patient compliance, and potentially reduce the frequency of administration. []
Q22: What are the most commonly reported adverse effects associated with repaglinide?
A: The most frequent adverse effects of repaglinide are hypoglycemia, upper respiratory tract infection, rhinitis, bronchitis, and headache. []
Q23: How does the risk of hypoglycemia with repaglinide compare to sulfonylureas?
A: Due to its shorter duration of action and meal-time dosing, repaglinide is associated with a lower risk of serious hypoglycemia compared to sulfonylureas, particularly if a meal is missed. [, , ]
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.
![N-[4-methoxy-3-(4-methylpiperazin-1-yl)phenyl]-1,2-dihydrobenzo[e]indole-3-carboxamide;hydrochloride](/img/structure/B1680439.png)
![N-[4-[(3aR,9bS)-8-cyano-3,3a,4,9b-tetrahydro-1H-chromeno[3,4-c]pyrrol-2-yl]butyl]-4-phenylbenzamide](/img/structure/B1680441.png)
![tert-butyl 2-[[(3S)-2-[(2S)-2-[3-[(4-nitro-2,1,3-benzoxadiazol-7-yl)amino]propanoylamino]-3-phenylpropanoyl]-1,2-oxazolidine-3-carbonyl]amino]acetate](/img/structure/B1680447.png)
![3-Bromo-4-(4-(2-methoxyphenyl)piperazin-1-yl)-1H-pyrazolo[3,4-d]pyrimidine](/img/structure/B1680452.png)
![N-[(2S)-1-[[(2S)-2-[[(2S,3S)-1-cyclohexyl-3-hydroxy-6-pyridin-2-ylhexan-2-yl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-1-oxo-3-phenylpropan-2-yl]-3-methylbutanamide](/img/structure/B1680457.png)
