Elvitegravir
Descripción general
Descripción
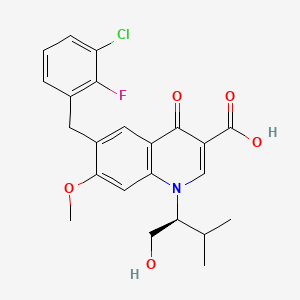
Elvitegravir is a quinolinemonocarboxylic acid that is 7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid substited at position 1 by a 1-hydroxy-3-methylbutan-2-yl group and at position 6 by a 3-chloro-2-fluorobenzyl group (the S-enantiomer). It is used in combination therapy for the treatment of HIV-1 infection. It has a role as a HIV-1 integrase inhibitor. It is a quinolinemonocarboxylic acid, an organofluorine compound, an aromatic ether, a quinolone and a member of monochlorobenzenes.
This compound is a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI) used for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults. Because integrase is necessary for viral replication, inhibition prevents the integration of HIV-1 DNA into the host genome and thereby blocks the formation of the HIV-1 provirus and resulting propagation of the viral infection. Although available as a single dose tablet, this compound must be used in combination with an HIV protease inhibitor coadministered with ritonavir and another antiretroviral drug. this compound was first licensed from Japan Tobacco in 2008 and developed by Gilead Sciences. It was FDA approved on August 27, 2012. On September 24, 2014, the FDA approved the single pill form of this compound.
This compound is a Human Immunodeficiency Virus Integrase Strand Transfer Inhibitor. The mechanism of action of this compound is as a HIV Integrase Inhibitor, and Cytochrome P450 2C9 Inducer.
This compound is a modified quinolone antibiotic with activity against human immunodeficiency virus 1. This compound is an inhibitor of viral integrase and retains activity against integrase mutants that are resistant to Raltegravir.
See also: ... View More ...
Aplicaciones Científicas De Investigación
Elvitegravir: Un Análisis Exhaustivo de las Aplicaciones de Investigación Científica
Tratamiento de la Infección por VIH-1: this compound se utiliza principalmente en combinación con otros medicamentos antirretrovirales para el tratamiento de la infección por VIH-1 en adultos que han recibido tratamiento anteriormente. Actúa como un inhibidor de la integrasa del VIH, bloqueando la capacidad del virus para integrar su material genético en el ADN de la célula huésped, lo cual es un paso crucial en el proceso de replicación viral .
Actividad Antiviral Contra Cepas de VIH: La investigación ha demostrado que this compound muestra una potente actividad antiviral no solo contra cepas de laboratorio del VIH-1, sino también contra cepas del VIH-2. Esta actividad de amplio espectro lo convierte en un componente valioso en la lucha contra diversas formas del virus del VIH .
Desarrollo y Caracterización de Fármacos: this compound ha sido objeto de una investigación exhaustiva para caracterizar sus propiedades y las impurezas relacionadas, lo cual es crucial para el control de calidad en el desarrollo de fármacos. Se han empleado técnicas como la espectroscopia de RMN y LC-MS para estudiar su estructura e identificar impurezas relacionadas con el proceso .
Farmacocinética y Desarrollo de Bioensayos: Se han realizado estudios para comprender la farmacocinética de this compound, lo cual es esencial para determinar su dosificación y frecuencia de administración. Además, se han desarrollado bioensayos utilizando polímeros impresos molecularmente para la extracción y medición específica de this compound, lo que ayuda en su análisis y garantía de calidad .
Ensayos Clínicos y Estudios Comparativos: this compound se ha evaluado en ensayos clínicos para evaluar su eficacia en comparación con otros inhibidores de la proteasa cuando se combina con terapia de fondo optimizada para individuos infectados con VIH-1. Estos estudios ayudan a establecer su lugar en los regímenes terapéuticos y guían las decisiones de tratamiento .
Investigación de Formulaciones: La investigación sobre diversas formulaciones de this compound, como diferentes prototipos de insertos para el tiempo de desintegración, el residuo, las fugas y la aceptabilidad, está en curso. Esta investigación tiene como objetivo optimizar los métodos de administración y mejorar el cumplimiento del paciente .
Mecanismo De Acción
Target of Action
Elvitegravir is an antiretroviral agent that primarily targets the HIV-1 integrase . This enzyme is encoded by the HIV-1 virus and is essential for viral replication .
Mode of Action
This compound acts as an integrase strand transfer inhibitor (INSTI) . The integrase enzyme is responsible for integrating the HIV-1 DNA into the host genome. By inhibiting this enzyme, this compound prevents the integration of HIV-1 DNA into the host genome, thereby blocking the formation of the HIV-1 provirus and the propagation of the viral infection .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the HIV-1 replication cycle . By inhibiting the integrase enzyme, this compound disrupts the integration of the viral DNA into the host genome, a crucial step in the HIV-1 replication cycle . This results in the prevention of the formation of new HIV-1 proviruses, thereby halting the propagation of the viral infection .
Pharmacokinetics
This compound undergoes primarily oxidative metabolism via CYP3A , and is secondarily glucuronidated via UGT1A1/3 enzymes . The metabolites of this compound are found in the plasma at very low concentrations and display considerably lower anti-HIV activity . This compound’s metabolism primarily occurs via cytochrome P450 3A4 (CYP3A4) and requires pharmacokinetic boosting to achieve systemic exposures amenable to once-daily dosing .
Result of Action
The molecular effect of this compound’s action is the inhibition of the HIV-1 integrase enzyme, which prevents the integration of HIV-1 DNA into the host genome . On a cellular level, this results in the blocking of the formation of the HIV-1 provirus and the propagation of the viral infection . This effectively halts the replication of the HIV-1 virus within the host cells .
Action Environment
Environmental factors such as the presence of other drugs can influence the action of this compound. For instance, this compound must be used in combination with an HIV protease inhibitor coadministered with ritonavir and another antiretroviral drug . Additionally, substances that induce CYP3A can reduce this compound concentrations in the body, potentially triggering the development of resistant virus strains . Furthermore, decreased levels of plasma albumin, which occur during pregnancy, can enhance the hepatic clearance of this compound, as it binds strongly to plasma albumin .
Propiedades
IUPAC Name |
6-[(3-chloro-2-fluorophenyl)methyl]-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxoquinoline-3-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H23ClFNO5/c1-12(2)19(11-27)26-10-16(23(29)30)22(28)15-8-14(20(31-3)9-18(15)26)7-13-5-4-6-17(24)21(13)25/h4-6,8-10,12,19,27H,7,11H2,1-3H3,(H,29,30)/t19-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JUZYLCPPVHEVSV-LJQANCHMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C(CO)N1C=C(C(=O)C2=C1C=C(C(=C2)CC3=C(C(=CC=C3)Cl)F)OC)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)[C@@H](CO)N1C=C(C(=O)C2=C1C=C(C(=C2)CC3=C(C(=CC=C3)Cl)F)OC)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H23ClFNO5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID101021650 | |
| Record name | Elvitegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101021650 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
447.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
<0.3 mcg/mL | |
| Record name | Elvitegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Elvitegravir is an HIV-1 integrase strand transfer inhibitor (INSTI). Integrase is an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the integration of HIV-1 DNA into host genomic DNA, blocking the formation of the HIV-1 provirus and propagation of the viral infection. Elvitegravir does not inhibit human topoisomerases I or II. | |
| Record name | Elvitegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
697761-98-1 | |
| Record name | Elvitegravir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=697761-98-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Elvitegravir [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0697761981 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Elvitegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Elvitegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101021650 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ELVITEGRAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4GDQ854U53 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.