6-Mercaptopurine
Vue d'ensemble
Description
Mécanisme D'action
Target of Action
Mercaptopurine, a purine analogue, primarily targets the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) . This enzyme plays a crucial role in the purine salvage pathway, which is essential for nucleotide synthesis and, consequently, DNA and RNA production .
Mode of Action
Mercaptopurine competes with hypoxanthine and guanine for HGPRTase and is converted into thioinosinic acid (TIMP) . This conversion disrupts the normal purine nucleotide interconversion, thereby inhibiting nucleic acid synthesis .
Biochemical Pathways
Mercaptopurine interferes with the purine metabolism pathway . It is converted into active metabolites, including thioguanine nucleotides (TGNs) , which incorporate into DNA and RNA, causing cell cycle arrest and apoptosis . The disruption of purine metabolism leads to a decrease in purine nucleotide pools and a halt in DNA and RNA synthesis, affecting cell proliferation .
Pharmacokinetics
The bioavailability of orally administered mercaptopurine varies significantly among individuals, ranging from 5 to 37% . It is metabolized by xanthine oxidase and its elimination half-life is between 60 to 120 minutes , but it’s longer for its active metabolites . The primary route of excretion is via the kidneys .
Result of Action
The incorporation of mercaptopurine’s metabolites into DNA and RNA disrupts their synthesis, leading to cell cycle arrest and apoptosis . This results in the death of rapidly dividing cells, particularly cancer cells, thereby inhibiting tumor growth .
Action Environment
The action of mercaptopurine can be influenced by various environmental factors. For instance, the concomitant use of allopurinol, a drug used to manage hyperuricemia, can alter mercaptopurine metabolism to maximize TGN production while reducing the hepatotoxic metabolite, 6-MMPN . Furthermore, individuals with a genetic deficiency in thiopurine S-methyltransferase, an enzyme involved in mercaptopurine metabolism, are at higher risk of side effects .
Applications De Recherche Scientifique
La 6-mercaptopurine a une large gamme d'applications en recherche scientifique :
5. Mécanisme d'Action
La this compound exerce ses effets en entrant en compétition avec l'hypoxanthine et la guanine pour l'enzyme hypoxanthine-guanine phosphoribosyltransférase (HGPRTase). Elle est convertie en acide thioinosinique, qui inhibe plusieurs réactions impliquant l'acide inosinique, telles que la conversion en acide xanthylique et en acide adénylique . Cette inhibition perturbe la synthèse de l'ADN et de l'ARN, conduisant à la mort cellulaire .
Composés Similaires :
Thioguanine : Un autre analogue de la purine utilisé dans le traitement de la leucémie.
Azathioprine : Un prod médicament qui est métabolisé en this compound et utilisé comme immunosuppresseur.
Allopurinol : Un inhibiteur de la xanthine oxydase qui peut interagir avec la this compound.
Unicité : La this compound est unique dans sa capacité à inhiber le métabolisme des purines et son double rôle d'agent antinéoplasique et immunosuppresseur . Contrairement à la thioguanine, qui est principalement utilisée pour ses propriétés antinéoplasiques, la this compound a des applications plus larges dans le traitement des maladies auto-immunes .
Analyse Biochimique
Biochemical Properties
Mercaptopurine interferes with nucleic acid biosynthesis by inhibiting purine metabolism . It interacts with various enzymes, proteins, and other biomolecules in the body, disrupting guanosine nucleotide homeostasis .
Cellular Effects
Mercaptopurine has significant effects on various types of cells and cellular processes. It influences cell function by impacting cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
The molecular mechanism of Mercaptopurine involves binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . It exerts its effects at the molecular level, leading to its antineoplastic and immunosuppressant properties .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Mercaptopurine change over time. Information on the product’s stability, degradation, and any long-term effects on cellular function observed in in vitro or in vivo studies is still being researched .
Dosage Effects in Animal Models
The effects of Mercaptopurine vary with different dosages in animal models. Studies have observed threshold effects, as well as toxic or adverse effects at high doses .
Metabolic Pathways
Mercaptopurine is involved in various metabolic pathways. It interacts with enzymes or cofactors, affecting metabolic flux or metabolite levels . It disrupts guanosine nucleotide homeostasis, which may contribute to its mechanism of action .
Subcellular Localization
This includes any targeting signals or post-translational modifications that direct it to specific compartments or organelles .
Méthodes De Préparation
Voies de Synthèse et Conditions de Réaction : La 6-mercaptopurine peut être synthétisée par plusieurs méthodes. Une méthode courante implique la réaction de la 4-amino-5-imidazolecarboxamide avec la thiourée en milieu acide pour obtenir la this compound . Une autre méthode implique la cyclisation de la 5-amino-1H-imidazole-4-carboxamide avec du disulfure de carbone suivie d'une réduction .
Méthodes de Production Industrielle : Dans les environnements industriels, la this compound est produite par une série de réactions chimiques impliquant la condensation de la 4-amino-5-imidazolecarboxamide avec la thiourée, suivie d'étapes de cyclisation et de purification . Le processus est optimisé pour garantir un rendement élevé et une pureté élevée du produit final.
Analyse Des Réactions Chimiques
Types de Réactions : La 6-mercaptopurine subit diverses réactions chimiques, notamment des réactions d'oxydation, de réduction et de substitution .
Réactifs et Conditions Courants :
Substitution : La this compound peut subir des réactions de substitution nucléophile avec des composés halogénés pour former divers dérivés.
Principaux Produits :
Oxydation : 6-Thioxanthine
Réduction : Dérivés réduits de la this compound
Substitution : Divers dérivés substitués de la this compound
Comparaison Avec Des Composés Similaires
Thioguanine: Another purine analogue used in the treatment of leukemia.
Azathioprine: A prodrug that is metabolized to mercaptopurine and used as an immunosuppressant.
Allopurinol: A xanthine oxidase inhibitor that can interact with mercaptopurine.
Uniqueness: Mercaptopurine is unique in its ability to inhibit purine metabolism and its dual role as an antineoplastic and immunosuppressant agent . Unlike thioguanine, which is primarily used for its antineoplastic properties, mercaptopurine has broader applications in treating autoimmune diseases .
Propriétés
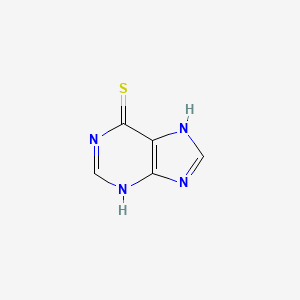
IUPAC Name |
3,7-dihydropurine-6-thione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C5H4N4S/c10-5-3-4(7-1-6-3)8-2-9-5/h1-2H,(H2,6,7,8,9,10) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GLVAUDGFNGKCSF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=NC2=C(N1)C(=S)N=CN2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C5H4N4S | |
| Record name | mercaptopurine | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Mercaptopurine | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
6112-76-1 (monohydrate) | |
| Record name | Mercaptopurine [USAN:USP:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050442 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID0020810 | |
| Record name | 6-Mercaptopurine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0020810 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
152.18 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Mercaptopurine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015167 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
22.5 [ug/mL] (The mean of the results at pH 7.4), In water, 6848 mg/L at 30 °C, Insoluble in water, Soluble in boiling water (1 in 100), Soluble in hot alcohol and dilute alkali solutions; slightly soluble in dilute sulfuric acid, Soluble in alkaline solutions (with decomposition), hot ethanol and ethanol (1 in 950); slightly soluble in dilute sulphuric acid; almost insoluble in water, acetone, chloroform and diethyl ether., 7.35e-01 g/L | |
| Record name | SID49675007 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Mercaptopurine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01033 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Mercaptopurine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3235 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mercaptopurine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015167 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Mercaptopurine competes with hypoxanthine and guanine for the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) and is itself converted to thioinosinic acid (TIMP). TIMP inhibits several reactions that involve inosinic acid (IMP), such as the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP). Upon methylation, TIMP forms 6-methylthioinosinate (MTIMP) which inhibits glutamine-5-phosphoribosylpyrophosphate amidotransferase in addition to TIMP. Glutamine-5-phosphoribosylpyrophosphate amidotransferase is the first enzyme unique to the _de novo_ pathway for purine ribonucleotide synthesis. According to experimental findings using radiolabeled mercaptopurine, mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine. In comparison, some mercaptopurine may be converted to nucleotide derivatives of 6-thioguanine (6-TG) via actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase that convert TIMP to thioguanylic acid (TGMP)., The pathogenesis of several neurodegenerative diseases often involves the microglial activation and associated inflammatory processes. Activated microglia release pro-inflammatory factors that may be neurotoxic. 6-Mercaptopurine (6-MP) is a well-established immunosuppressive drug. Common understanding of their immunosuppressive properties is largely limited to peripheral immune cells. However, the effect of 6-MP in the central nervous system, especially in microglia in the context of neuroinflammation is, as yet, unclear. Tumor necrosis factor-alpha (TNF-a) is a key cytokine of the immune system that initiates and promotes neuroinflammation. The present study aimed to investigate the effect of 6-MP on TNF-a production by microglia to discern the molecular mechanisms of this modulation. Lipopolysaccharide (LPS) was used to induce an inflammatory response in cultured primary microglia or murine BV-2 microglial cells. Released TNF-a was measured by enzyme-linked immunosorbent assay (ELISA). Gene expression was determined by real-time reverse transcription polymerase chain reaction (RT-PCR). Signaling molecules were analyzed by western blotting, and activation of NF-kB was measured by ELISA-based DNA binding analysis and luciferase reporter assay. Chromatin immunoprecipitation (ChIP) analysis was performed to examine NF-kB p65 and coactivator p300 enrichments and histone modifications at the endogenous TNF-a promoter. Treatment of LPS-activated microglia with 6-MP significantly attenuated TNF-a production. In 6-MP pretreated microglia, LPS-induced MAPK signaling, I?B-a degradation, NF-kB p65 nuclear translocation, and in vitro p65 DNA binding activity were not impaired. However, 6-MP suppressed transactivation activity of NF-?B and TNF-a promoter by inhibiting phosphorylation and acetylation of p65 on Ser276 and Lys310, respectively. ChIP analyses revealed that 6-MP dampened LPS-induced histone H3 acetylation of chromatin surrounding the TNF-a promoter, ultimately leading to a decrease in p65/coactivator-mediated transcription of TNF-a gene. Furthermore, 6-MP enhanced orphan nuclear receptor Nur77 expression. Using RNA interference approach, we further demonstrated that Nur77 upregulation contribute to 6-MP-mediated inhibitory effect on TNF-a production. Additionally, 6-MP also impeded TNF-a mRNA translation through prevention of LPS-activated PI3K/Akt/mTOR signaling cascades. These results suggest that 6-MP might have a therapeutic potential in neuroinflammation-related neurodegenerative disorders through downregulation of microglia-mediated inflammatory processes., Mercaptopurine (6-MP) competes with hypoxanthine and guanine for the enzyme hyphoxanthine-guanine phosphoribosyltransferase (HGPRTase) and is itself converted to thioinosinic acid (TIMP). This intracellular nucleotide inhibits several reactions involving inosinic acid (IMP), including the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP). In addition, 6-methylthioinosinate (MTIMP) is formed by the methylation of TIMP. Both TIMP and MTIMP have been reported to inhibit glutamine-5-phosphoribosylpyrophosphate amidotransferase, the first enzyme unique to the de novo pathway for purine ribonucleotide synthesis. Experiments indicate that radiolabeled mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine. Some mercaptopurine is converted to nucleotide derivatives of 6-thioguanine (6-TG) by the sequential actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase, converting TIMP to thioguanylic acid (TGMP). Animal tumors that are resistant to mercaptopurine often have lost the ability to convert mercaptopurine to TIMP. However, it is clear that resistance to mercaptopurine may be acquired by other means as well, particularly in human leukemias. It is not known exactly which of any one or more of the biochemical effects of mercaptopurine and its metabolites are directly or predominantly responsible for cell death., Inflammatory bowel disease is characterized by chronic intestinal inflammation. Azathioprine and its metabolite 6-mercaptopurine (6-MP) are effective immunosuppressive drugs that are widely used in patients with inflammatory bowel disease. ... Azathioprine and 6-MP have been shown to affect small GTPase Rac1 in T cells and endothelial cells, whereas the effect on macrophages and gut epithelial cells is unknown. Macrophages (RAW cells) and gut epithelial cells (Caco-2 cells) were activated by cytokines and the effect on Rac1 signaling was assessed in the presence or absence of 6-MP. Rac1 is activated in macrophages and epithelial cells, and treatment with 6-MP resulted in Rac1 inhibition. In macrophages, interferon-gamma induced downstream signaling through c-Jun-N-terminal Kinase (JNK) resulting in inducible nitric oxide synthase (iNOS) expression. iNOS expression was reduced by 6-MP in a Rac1-dependent manner. In epithelial cells, 6-MP efficiently inhibited tumor necrosis factor-a-induced expression of the chemokines CCL2 and interleukin-8, although only interleukin-8 expression was inhibited in a Rac1-dependent manner. In addition, activation of the transcription factor STAT3 was suppressed in a Rac1-dependent fashion by 6-MP, resulting in reduced proliferation of the epithelial cells due to diminished cyclin D1 expression. These data demonstrate that 6-MP affects macrophages and gut epithelial cells beneficially, in addition to T cells and endothelial cells. Furthermore, mechanistic insight is provided to support development of Rac1-specific inhibitors for clinical use in inflammatory bowel disease. | |
| Record name | Mercaptopurine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01033 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Mercaptopurine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3235 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Yellow crystalline powder, Yellow prisms from water (+1 water), Dark yellow /Mercaptopurine hydrate/ | |
CAS No. |
50-44-2, 6112-76-1 | |
| Record name | 6-Mercaptopurine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-44-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Mercaptopurine [USAN:USP:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050442 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Mercaptopurine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01033 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | mercaptopurine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759614 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 6-Mercaptopurine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=755 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 6-Mercaptopurine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0020810 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1,7-dihydro-6H-purine-6-thione hydrate (1:1) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.126.167 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Mercaptopurine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.035 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MERCAPTOPURINE ANHYDROUS | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/PKK6MUZ20G | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Mercaptopurine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3235 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mercaptopurine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015167 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
308 °C (decomposes), 313 °C | |
| Record name | Mercaptopurine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01033 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Mercaptopurine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3235 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mercaptopurine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015167 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.