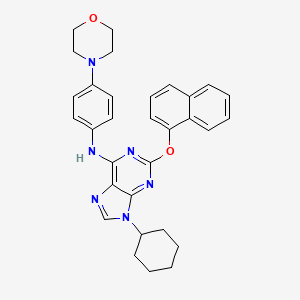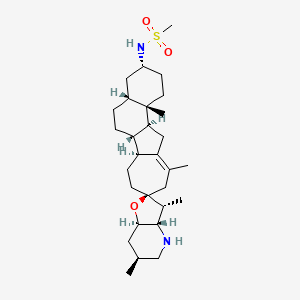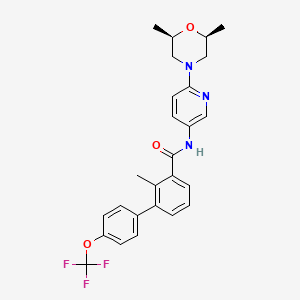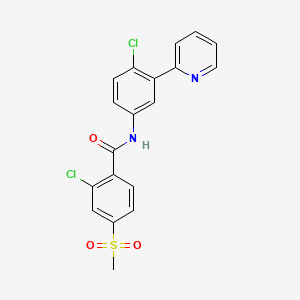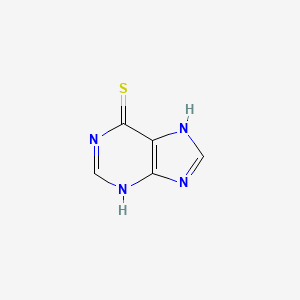
6-メルカプトプリン
概要
説明
作用機序
メルカプトプリンは、ヒポキサンチン-グアニンホスホリボシルトランスフェラーゼ(HGPRTase)酵素に対してヒポキサンチンやグアニンと競合することによって効果を発揮します。 メルカプトプリンはチオイノシン酸に変換され、イノシン酸からキサンチル酸やアデニル酸への変換などの、イノシン酸に関与するいくつかの反応を阻害します . この阻害はDNAとRNA合成を阻害し、細胞死をもたらします .
類似化合物:
チオグアニン: 白血病の治療に使用される別のプリン類似体です.
アザチオプリン: メルカプトプリンに代謝されるプロドラッグで、免疫抑制剤として使用されます.
アロプリノール: メルカプトプリンと相互作用する可能性のあるキサンチンオキシダーゼ阻害剤です.
独自性: メルカプトプリンは、プリン代謝を阻害する能力と、抗悪性腫瘍剤および免疫抑制剤としての二重の役割においてユニークです . 主に抗悪性腫瘍特性のために使用されるチオグアニンとは異なり、メルカプトプリンは自己免疫疾患の治療においてより幅広い用途を持っています .
科学的研究の応用
Mercaptopurine has a wide range of applications in scientific research:
Chemistry: Used as a model compound to study purine metabolism and nucleic acid synthesis.
Medicine: Widely used in the treatment of acute lymphocytic leukemia, Crohn’s disease, and ulcerative colitis.
Industry: Utilized in the development of new pharmaceuticals and as a reference standard in quality control.
生化学分析
Biochemical Properties
Mercaptopurine interferes with nucleic acid biosynthesis by inhibiting purine metabolism . It interacts with various enzymes, proteins, and other biomolecules in the body, disrupting guanosine nucleotide homeostasis .
Cellular Effects
Mercaptopurine has significant effects on various types of cells and cellular processes. It influences cell function by impacting cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
The molecular mechanism of Mercaptopurine involves binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . It exerts its effects at the molecular level, leading to its antineoplastic and immunosuppressant properties .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Mercaptopurine change over time. Information on the product’s stability, degradation, and any long-term effects on cellular function observed in in vitro or in vivo studies is still being researched .
Dosage Effects in Animal Models
The effects of Mercaptopurine vary with different dosages in animal models. Studies have observed threshold effects, as well as toxic or adverse effects at high doses .
Metabolic Pathways
Mercaptopurine is involved in various metabolic pathways. It interacts with enzymes or cofactors, affecting metabolic flux or metabolite levels . It disrupts guanosine nucleotide homeostasis, which may contribute to its mechanism of action .
Subcellular Localization
This includes any targeting signals or post-translational modifications that direct it to specific compartments or organelles .
準備方法
合成経路と反応条件: メルカプトプリンは、いくつかの方法で合成することができます。 一般的な方法の1つは、酸性条件下で4-アミノ-5-イミダゾールカルボキサミドとチオ尿素を反応させて6-メルカプトプリンを得ることです . 別の方法には、5-アミノ-1H-イミダゾール-4-カルボキサミドと二硫化炭素を環化し、続いて還元する反応が含まれます .
工業生産方法: 産業的には、メルカプトプリンは、4-アミノ-5-イミダゾールカルボキサミドとチオ尿素を縮合させ、続いて環化および精製工程を行う一連の化学反応によって製造されます . このプロセスは、最終生成物の高収率と純度を確保するために最適化されています。
化学反応の分析
反応の種類: メルカプトプリンは、酸化、還元、置換反応を含むさまざまな化学反応を起こします .
一般的な試薬と条件:
酸化: 過酸化水素や過マンガン酸カリウムなどの酸化剤を使用して、メルカプトプリンを酸化して6-チオキサンチンを生成することができます.
主要な生成物:
酸化: 6-チオキサンチン
還元: 還元されたメルカプトプリン誘導体
置換: さまざまな置換されたメルカプトプリン誘導体
4. 科学研究への応用
メルカプトプリンは、科学研究において幅広い応用範囲を持っています。
類似化合物との比較
Thioguanine: Another purine analogue used in the treatment of leukemia.
Azathioprine: A prodrug that is metabolized to mercaptopurine and used as an immunosuppressant.
Allopurinol: A xanthine oxidase inhibitor that can interact with mercaptopurine.
Uniqueness: Mercaptopurine is unique in its ability to inhibit purine metabolism and its dual role as an antineoplastic and immunosuppressant agent . Unlike thioguanine, which is primarily used for its antineoplastic properties, mercaptopurine has broader applications in treating autoimmune diseases .
特性
IUPAC Name |
3,7-dihydropurine-6-thione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C5H4N4S/c10-5-3-4(7-1-6-3)8-2-9-5/h1-2H,(H2,6,7,8,9,10) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GLVAUDGFNGKCSF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=NC2=C(N1)C(=S)N=CN2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C5H4N4S | |
| Record name | mercaptopurine | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Mercaptopurine | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
6112-76-1 (monohydrate) | |
| Record name | Mercaptopurine [USAN:USP:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050442 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID0020810 | |
| Record name | 6-Mercaptopurine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0020810 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
152.18 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Mercaptopurine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015167 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
22.5 [ug/mL] (The mean of the results at pH 7.4), In water, 6848 mg/L at 30 °C, Insoluble in water, Soluble in boiling water (1 in 100), Soluble in hot alcohol and dilute alkali solutions; slightly soluble in dilute sulfuric acid, Soluble in alkaline solutions (with decomposition), hot ethanol and ethanol (1 in 950); slightly soluble in dilute sulphuric acid; almost insoluble in water, acetone, chloroform and diethyl ether., 7.35e-01 g/L | |
| Record name | SID49675007 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Mercaptopurine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01033 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Mercaptopurine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3235 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mercaptopurine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015167 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Mercaptopurine competes with hypoxanthine and guanine for the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) and is itself converted to thioinosinic acid (TIMP). TIMP inhibits several reactions that involve inosinic acid (IMP), such as the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP). Upon methylation, TIMP forms 6-methylthioinosinate (MTIMP) which inhibits glutamine-5-phosphoribosylpyrophosphate amidotransferase in addition to TIMP. Glutamine-5-phosphoribosylpyrophosphate amidotransferase is the first enzyme unique to the _de novo_ pathway for purine ribonucleotide synthesis. According to experimental findings using radiolabeled mercaptopurine, mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine. In comparison, some mercaptopurine may be converted to nucleotide derivatives of 6-thioguanine (6-TG) via actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase that convert TIMP to thioguanylic acid (TGMP)., The pathogenesis of several neurodegenerative diseases often involves the microglial activation and associated inflammatory processes. Activated microglia release pro-inflammatory factors that may be neurotoxic. 6-Mercaptopurine (6-MP) is a well-established immunosuppressive drug. Common understanding of their immunosuppressive properties is largely limited to peripheral immune cells. However, the effect of 6-MP in the central nervous system, especially in microglia in the context of neuroinflammation is, as yet, unclear. Tumor necrosis factor-alpha (TNF-a) is a key cytokine of the immune system that initiates and promotes neuroinflammation. The present study aimed to investigate the effect of 6-MP on TNF-a production by microglia to discern the molecular mechanisms of this modulation. Lipopolysaccharide (LPS) was used to induce an inflammatory response in cultured primary microglia or murine BV-2 microglial cells. Released TNF-a was measured by enzyme-linked immunosorbent assay (ELISA). Gene expression was determined by real-time reverse transcription polymerase chain reaction (RT-PCR). Signaling molecules were analyzed by western blotting, and activation of NF-kB was measured by ELISA-based DNA binding analysis and luciferase reporter assay. Chromatin immunoprecipitation (ChIP) analysis was performed to examine NF-kB p65 and coactivator p300 enrichments and histone modifications at the endogenous TNF-a promoter. Treatment of LPS-activated microglia with 6-MP significantly attenuated TNF-a production. In 6-MP pretreated microglia, LPS-induced MAPK signaling, I?B-a degradation, NF-kB p65 nuclear translocation, and in vitro p65 DNA binding activity were not impaired. However, 6-MP suppressed transactivation activity of NF-?B and TNF-a promoter by inhibiting phosphorylation and acetylation of p65 on Ser276 and Lys310, respectively. ChIP analyses revealed that 6-MP dampened LPS-induced histone H3 acetylation of chromatin surrounding the TNF-a promoter, ultimately leading to a decrease in p65/coactivator-mediated transcription of TNF-a gene. Furthermore, 6-MP enhanced orphan nuclear receptor Nur77 expression. Using RNA interference approach, we further demonstrated that Nur77 upregulation contribute to 6-MP-mediated inhibitory effect on TNF-a production. Additionally, 6-MP also impeded TNF-a mRNA translation through prevention of LPS-activated PI3K/Akt/mTOR signaling cascades. These results suggest that 6-MP might have a therapeutic potential in neuroinflammation-related neurodegenerative disorders through downregulation of microglia-mediated inflammatory processes., Mercaptopurine (6-MP) competes with hypoxanthine and guanine for the enzyme hyphoxanthine-guanine phosphoribosyltransferase (HGPRTase) and is itself converted to thioinosinic acid (TIMP). This intracellular nucleotide inhibits several reactions involving inosinic acid (IMP), including the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP). In addition, 6-methylthioinosinate (MTIMP) is formed by the methylation of TIMP. Both TIMP and MTIMP have been reported to inhibit glutamine-5-phosphoribosylpyrophosphate amidotransferase, the first enzyme unique to the de novo pathway for purine ribonucleotide synthesis. Experiments indicate that radiolabeled mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine. Some mercaptopurine is converted to nucleotide derivatives of 6-thioguanine (6-TG) by the sequential actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase, converting TIMP to thioguanylic acid (TGMP). Animal tumors that are resistant to mercaptopurine often have lost the ability to convert mercaptopurine to TIMP. However, it is clear that resistance to mercaptopurine may be acquired by other means as well, particularly in human leukemias. It is not known exactly which of any one or more of the biochemical effects of mercaptopurine and its metabolites are directly or predominantly responsible for cell death., Inflammatory bowel disease is characterized by chronic intestinal inflammation. Azathioprine and its metabolite 6-mercaptopurine (6-MP) are effective immunosuppressive drugs that are widely used in patients with inflammatory bowel disease. ... Azathioprine and 6-MP have been shown to affect small GTPase Rac1 in T cells and endothelial cells, whereas the effect on macrophages and gut epithelial cells is unknown. Macrophages (RAW cells) and gut epithelial cells (Caco-2 cells) were activated by cytokines and the effect on Rac1 signaling was assessed in the presence or absence of 6-MP. Rac1 is activated in macrophages and epithelial cells, and treatment with 6-MP resulted in Rac1 inhibition. In macrophages, interferon-gamma induced downstream signaling through c-Jun-N-terminal Kinase (JNK) resulting in inducible nitric oxide synthase (iNOS) expression. iNOS expression was reduced by 6-MP in a Rac1-dependent manner. In epithelial cells, 6-MP efficiently inhibited tumor necrosis factor-a-induced expression of the chemokines CCL2 and interleukin-8, although only interleukin-8 expression was inhibited in a Rac1-dependent manner. In addition, activation of the transcription factor STAT3 was suppressed in a Rac1-dependent fashion by 6-MP, resulting in reduced proliferation of the epithelial cells due to diminished cyclin D1 expression. These data demonstrate that 6-MP affects macrophages and gut epithelial cells beneficially, in addition to T cells and endothelial cells. Furthermore, mechanistic insight is provided to support development of Rac1-specific inhibitors for clinical use in inflammatory bowel disease. | |
| Record name | Mercaptopurine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01033 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Mercaptopurine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3235 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Yellow crystalline powder, Yellow prisms from water (+1 water), Dark yellow /Mercaptopurine hydrate/ | |
CAS No. |
50-44-2, 6112-76-1 | |
| Record name | 6-Mercaptopurine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-44-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Mercaptopurine [USAN:USP:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050442 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Mercaptopurine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01033 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | mercaptopurine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759614 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 6-Mercaptopurine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=755 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 6-Mercaptopurine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0020810 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1,7-dihydro-6H-purine-6-thione hydrate (1:1) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.126.167 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Mercaptopurine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.035 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MERCAPTOPURINE ANHYDROUS | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/PKK6MUZ20G | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Mercaptopurine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3235 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mercaptopurine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015167 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
308 °C (decomposes), 313 °C | |
| Record name | Mercaptopurine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01033 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Mercaptopurine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3235 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mercaptopurine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015167 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。