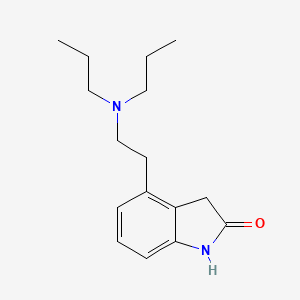
ロピニロール
概要
説明
ロピニロールは、主にパーキンソン病とレストレスレッグス症候群の治療に使用される非エルゴライン系ドーパミン作動薬です。米国では1997年に初めて医療用として承認されました。 ロピニロールは、脳内のドーパミンD2受容体を刺激することにより作用し、ドーパミン欠乏に関連する症状の緩和に役立ちます .
製法
合成経路と反応条件
ロピニロールは、いくつかの方法で合成することができます。一般的な方法の1つは、2-ニトロフェニル酢酸前駆体の還元とそれに続く自発的な環化です。 別の方法には、触媒としてパラジウム炭素(Pd/C)を使用する2-(2'-ブロモエチル)β-ニトロスチレンの還元環化が含まれます .
工業的製造方法
工業環境では、ロピニロール塩酸塩は、通常、エタノール、メタノール、または酢酸エチルなどの溶媒に化合物を溶解し、続いてPd/Cを添加することによって調製されます。 次に、混合物を反応させて所望の生成物を得ます .
作用機序
ロピニロールは、脳の尾状核-被殻系内のドーパミンD2受容体を選択的に刺激することで効果を発揮します。この刺激は、パーキンソン病などの疾患で観察されるドーパミン欠乏を補うのに役立ちます。 ロピニロールは、D2およびD3受容体に対して高い親和性を示し、D3受容体に対してはより高い親和性を示します .
類似の化合物との比較
類似の化合物
プラミペキソール: パーキンソン病とレストレスレッグス症候群の治療に使用されるもう1つの非エルゴライン系ドーパミン作動薬です。
独自性
ロピニロールは、他のドーパミン作動薬と比較して、D3受容体に対する高い親和性を持つ点が特徴です。 この特異性は、パーキンソン病とレストレスレッグス症候群の症状の治療における有効性に貢献している可能性があります .
科学的研究の応用
Ropinirole has a wide range of scientific research applications:
生化学分析
Biochemical Properties
Ropinirole plays a crucial role in biochemical reactions by acting as a dopamine receptor agonist. It specifically targets dopamine D2 and D3 receptors, with a higher affinity for D3 receptors . By binding to these receptors, ropinirole stimulates dopaminergic activity in the brain, which helps alleviate symptoms of Parkinson’s disease and Restless Legs Syndrome . The interaction with these receptors is essential for its therapeutic effects.
Cellular Effects
Ropinirole influences various cellular processes, particularly in neurons. It enhances dopaminergic signaling, which is vital for motor control and coordination . This compound also affects gene expression by modulating the activity of dopamine receptors, leading to changes in cellular metabolism and function . Additionally, ropinirole has been shown to protect neurons from apoptosis and reduce inflammation in certain cellular models .
Molecular Mechanism
At the molecular level, ropinirole exerts its effects by binding to dopamine D2 and D3 receptors, which are G-protein-coupled receptors . This binding activates intracellular signaling pathways, including the inhibition of adenylate cyclase and the modulation of ion channels . These actions result in increased dopaminergic activity, which helps improve motor function and reduce symptoms of Parkinson’s disease and Restless Legs Syndrome .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of ropinirole have been observed to change over time. Studies have shown that ropinirole is stable under standard conditions but can degrade under extreme conditions . Long-term exposure to ropinirole has been associated with sustained improvements in motor function and reduced “off-time” in patients with Parkinson’s disease . Prolonged use can also lead to tolerance and the need for higher doses to achieve the same therapeutic effects .
Dosage Effects in Animal Models
In animal models, the effects of ropinirole vary with different dosages. Low doses of ropinirole have been shown to improve motor function and reduce symptoms of Parkinson’s disease . High doses can lead to adverse effects such as dyskinesia and compulsive behaviors . Studies have also indicated a threshold effect, where doses above a certain level do not provide additional therapeutic benefits and may increase the risk of side effects .
Metabolic Pathways
Ropinirole is metabolized primarily in the liver by the cytochrome P450 enzyme CYP1A2 . The major metabolic pathways include N-despropylation and hydroxylation, resulting in the formation of inactive metabolites . These metabolites are then excreted through the kidneys. The metabolism of ropinirole can be influenced by other drugs that affect CYP1A2 activity, leading to potential drug interactions .
Transport and Distribution
Ropinirole is widely distributed throughout the body, with a volume of distribution of approximately 7.5 L/kg . It is about 40% bound to plasma proteins and has a blood-to-plasma ratio of 1:1 . The compound is rapidly absorbed and extensively distributed from the vascular compartment to various tissues . The transport and distribution of ropinirole are crucial for its therapeutic effects, as it needs to reach the brain to exert its dopaminergic activity .
Subcellular Localization
The subcellular localization of ropinirole is primarily within the cytoplasm and at the cell membrane, where it interacts with dopamine receptors . The compound’s localization is influenced by its lipophilicity and the presence of specific transporters that facilitate its entry into cells . Ropinirole’s activity is dependent on its ability to reach and bind to dopamine receptors, which are predominantly located on the cell membrane .
準備方法
Synthetic Routes and Reaction Conditions
Ropinirole can be synthesized through several methods. One common method involves the reduction of a 2-nitrophenyl acetic acid precursor followed by spontaneous cyclization. Another method involves the reductive cyclization of 2-(2’-bromoethyl) β-nitrostyrene using palladium on carbon (Pd/C) as a catalyst .
Industrial Production Methods
In industrial settings, ropinirole hydrochloride is often prepared by dissolving the compound in solvents such as ethanol, methanol, or ethyl acetate, followed by the addition of Pd/C. The mixture is then reacted to obtain the desired product .
化学反応の分析
反応の種類
ロピニロールは、次のようなさまざまな化学反応を起こします。
酸化: ロピニロールは、酸化されてさまざまな代謝産物を形成することができます。
還元: ニトロ基をアミンに還元することは、その合成における重要なステップです。
一般的な試薬と条件
酸化: 一般的な酸化剤には、過酸化水素と過マンガン酸カリウムが含まれます。
還元: パラジウム炭素(Pd/C)などの触媒が、還元環化に使用されます。
生成される主な生成物
これらの反応から生成される主な生成物には、最終的にロピニロール塩酸塩の形成につながるさまざまな中間体が含まれます .
科学研究の応用
ロピニロールは、さまざまな科学研究の応用があります。
類似化合物との比較
Similar Compounds
Pramipexole: Another non-ergoline dopamine agonist used in the treatment of Parkinson’s disease and Restless Legs Syndrome.
Cabergoline: A dopamine agonist used primarily for the treatment of hyperprolactinemia but also has applications in Parkinson’s disease.
Uniqueness
Ropinirole is unique in its high affinity for D3 receptors compared to other dopamine agonists. This specificity may contribute to its effectiveness in treating symptoms of Parkinson’s disease and Restless Legs Syndrome .
特性
IUPAC Name |
4-[2-(dipropylamino)ethyl]-1,3-dihydroindol-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H24N2O/c1-3-9-18(10-4-2)11-8-13-6-5-7-15-14(13)12-16(19)17-15/h5-7H,3-4,8-12H2,1-2H3,(H,17,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UHSKFQJFRQCDBE-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCN(CCC)CCC1=C2CC(=O)NC2=CC=C1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H24N2O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
91374-20-8 (hydrochloride) | |
| Record name | Ropinirole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0091374219 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID8045195 | |
| Record name | Ropinirole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8045195 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
260.37 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
410.5±45.0 °C at 760 mmHg | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Solubility |
3.53e-01 g/L | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Ropinirole is a non-ergoline dopamine agonist. Ropinirole has the highest affinity at the D3 receptors, which are concentrated in the limbic areas of the brain and may be responsible for some of the neuropsychiatric effects. The exact mechanism of action of ropinirole as a treatment for Parkinson’s disease is unknown, however, it is believed to be related to its ability to selectively stimulate dopamine D2 receptors within the caudate-putamen system in the brain. This system affects body movement. Negligible affinity is seen for ropinirole at α2 adrenoreceptors in the periphery and 5HT-1 receptor. Ropinirole has no affinity at the D1-like receptors, benzodiazepine or GABA receptors. The precise mechanism of action of ropinirole as a treatment for Restless Legs Syndrome is unknown, however, it is believed to be related to its ability to stimulate dopamine receptors., The present study determined its affinity and agonist efficacy at recombinant human (h) dopamine hD2, hD3 and hD4 and serotonin (5-HT) h5-HT1A, h5-HT1B and h5-HT1D receptors. Roxindole exhibited high affinity at hD3 as well as at hD2 (short isoform) and hD4 (4-repeat isoform) receptors (pKi values 8.93, 8.55 and 8.23, respectively). Further, it displayed high affinity at hS-HT1A receptors (pKi = 9.42) but modest affinity at 5-HT1B and 5-HT1D receptors (pKi values 6.00 and 7.05, respectively). In [35S]GTPgammaS binding experiments, roxindole was >20-fold more potent in stimulating [35S]GTPgammaS binding at hD3 than at hD2 or hD4 receptors (pEC50 = 9.23 vs. 7.88 and 7.69). However, whereas roxindole exhibited partial agonist activity at hD3 and hD4 sites (Emax = 30.0% and 35.1%, respectively, relative to dopamine = 100%), it only weakly activated hD2 receptors (Emax = 10.5%). Roxindole potently blocked dopamine-stimulated [35S]GTPgammaS binding at hD2 receptors (pkappaB = 9.05). In comparison, the dopamine receptor agonist, (-)quinpirole, acted as a partial agonist at hD3 and hD4 sites (Emax = 67.4% and 66.3%, respectively) but surpassed the efficacy of dopamine at hD2 receptors (Emax = 132%). At h5-HT1A receptors, roxindole behaved as a high affinity (pKi = 9.42) partial agonist (Emax = 59.6%, relative to 5-HT = 100%), whereas (-)quinpirole had negligible activity. The selective 5-HT1A antagonist, WAY 100,635, blocked roxindole (100 nM)-stimulated [35S]GTPgammaS binding at h5-HT1A receptors in a concentration-dependent manner (pkappaB = 9.28). Roxindole only weakly stimulated [35S]GTPgammaS binding at 5-HT1B and 5-HT1D receptors (Emax = 27.1% and 13.7%). The present data suggest that roxindole activates mainly D3 vs. D2 or D4 receptors and 5-HT1A vs. 5-HT1B or 5-HT1D receptors. Activation of D3 and/or 5-HT1A receptors may thus contribute to its potential antidepressant properties., The aim of the present study was to characterize functional responses to ropinirole, its major metabolites in man (SKF-104557 (4-[2-(propylamino)ethyl]-2-(3H) indolone), SKF-97930 (4-carboxy-2-(3H) indolone)) and other dopamine receptor agonists at human dopamine D2(long) (hD2), D3 (hD3) and D4.4 (hD4) receptors separately expressed in Chinese hamster ovary cells using microphysiometry. 2. All the receptor agonists tested (ropinirole, SKF-104557, SKF-97930, bromocriptine, lisuride, pergolide, pramipexole, talipexole, dopamine) increased extracellular acidification rate in Chinese hamster ovary clones expressing the human D2, D3 or D4 receptor. The pEC50s of ropinirole at hD2, hD3 and hD4 receptors were 7.4, 8.4 and 6.8, respectively. Ropinirole is therefore at least 10 fold selective for the human dopamine D3 receptor over the other D2 receptor family members. 3. At the hD2 and hD3 dopamine receptors all the compounds tested were full agonists as compared to quinpirole. Talipexole and the ropinirole metabolite, SKF-104557, were partial agonists at the hD4 receptor. 4. Bromocriptine and lisuride had a slow onset of agonist action which precluded determination of EC50s. 5. The rank order of agonist potencies was dissimilar to the rank order of radioligand binding affinities at each of the dopamine receptor subtypes. Functional selectivities of the dopamine receptor agonists, as measured in the microphysiometer, were less than radioligand binding selectivities. 6. The results show that ropinirole is a full agonist at human D2, D3 and D4 dopamine receptors. SKF-104557 the major human metabolite of ropinirole, had similar radioligand binding affinities to, but lower functional potencies than, the parent compound., Ropinirole, which is a non-ergot dopamine agonist derivative, exerts therapeutic benefits in Parkinson's disease (PD). Based on recent studies implicating dopamine receptors 2 and 3 (D2R and D3R) as possible targets of ropinirole, we over-expressed these dopamine receptor genes in the dopamine-denervated striatum of rodents to reveal whether their over-expression modulated ropinirole activity. Adult Sprague-Dawley rats initially received unilateral 6-hydroxydopamine lesion of the medial forebrain bundle. At 1 month after surgery, successfully lesioned animals (3 or less forelimb akinesia score, and 8 or more apomorphine-induced rotations/min over 1 hr) were randomly assigned to intrastriatal injection (ipsilateral to the lesion) of blank lentiviral vector, D2R, D3R or both genes. At about 5 months post-lesion, ropinirole (0.2 mg/kg, i.p.) was administered daily for 9 consecutive days. The subtherapeutic dose of ropinirole improved the use of previously akinetic forelimb and produced robust circling behavior in lesioned animals with striatal over-expression of both D2R and D3R compared to lesioned animals that received blank vector. In contrast, the subtherapeutic dose of ropinirole generated only modest motor effects in lesioned animals with sole over-expression of D2R or D3R. Western immunoblot and autoradiographic assays showed enhanced D2R and D3R protein levels coupled with normalized D2R and D3R binding in the ventral striatum of lesioned animals with lentiviral over-expression of both D2R and D3R relative to vehicle-treated lesioned animals. Immunohistochemical analyses showed that D2R and D3R GFP fluorescent cells colocalized with enkephalin and substance P immunoreactive medium spiny neurons. These data support the use of the subtherapeutic dose of ropinirole in a chronic model of PD., Ropinirole hydrochloride, a dipropylaminoethyl indolone derivative, is a nonergot-derivative dopamine receptor agonist. In in vitro binding studies, ropinirole demonstrated high binding specificity for and intrinsic activity at dopamine D2 receptors compared with other dopamine receptor agonists (e.g., bromocriptine, pergolide), having a higher affinity for the D3 subtype than for the D2 or D4 subtypes. Ropinirole binds with moderate affinity to opiate receptors but has little or no affinity for alpha1-, alpha2-, or beta-adrenergic; dopamine D1; benzodiazepine; gamma-aminobutyric acid (GABA); serotonin type 1 (5-hydroxytryptamine (5-HT1)); serotonin type 2 (5-HT2); or muscarinic receptors. | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ROPINIROLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
91374-21-9 | |
| Record name | Ropinirole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=91374-21-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ropinirole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0091374219 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ropinirole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758917 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Ropinirole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8045195 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2H-Indol-2-one, 4-[2-(dipropylamino)ethyl]-1,3-dihydro | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.110.353 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ROPINIROLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/030PYR8953 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | ROPINIROLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
243-250 °C, 243 - 250 °C | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does ropinirole interact with its target?
A: Ropinirole is a non-ergoline dopamine agonist that primarily targets dopamine D2-like receptors, exhibiting a high affinity for both D2 and D3 subtypes. [, , , ] It mimics the action of dopamine by binding to these receptors, leading to downstream signaling events.
Q2: What are the downstream effects of ropinirole binding to dopamine receptors?
A: The binding of ropinirole to D2-like receptors, particularly in the striatum, helps restore dopaminergic signaling, which is deficient in Parkinson’s disease. [, , ] This, in turn, helps alleviate motor symptoms such as tremors, rigidity, and bradykinesia.
Q3: What is the molecular formula and weight of ropinirole?
A3: Ropinirole has the molecular formula C16H24N2O2 and a molecular weight of 276.37 g/mol.
Q4: Is there any spectroscopic data available for ropinirole?
A: While the provided research articles do not delve into detailed spectroscopic data, studies often employ techniques like UV spectrophotometry and RP-HPLC for ropinirole analysis. []
Q5: What are the different formulations of ropinirole available?
A: Ropinirole is available in both immediate-release and 24-hour prolonged-release formulations. [, , , , , ] The prolonged-release formulation aims to maintain more consistent dopaminergic activity with steadier plasma levels, potentially leading to improved tolerability and compliance.
Q6: How is ropinirole absorbed and distributed in the body?
A: Ropinirole is rapidly and almost completely absorbed after oral administration. [, , ] It exhibits good distribution throughout the body, crossing the blood-brain barrier to reach its target sites in the brain. []
Q7: What is the primary route of ropinirole metabolism?
A: Ropinirole is extensively metabolized in the liver, primarily by the cytochrome P450 (CYP) isoenzyme CYP1A2. [, , ] It also undergoes metabolism by CYP3A4/5 to a lesser extent. []
Q8: How is ropinirole eliminated from the body?
A: The primary route of ropinirole elimination is renal excretion, with approximately 60-90% of the administered dose being eliminated through urine. []
Q9: Does the presence of other drugs affect ropinirole pharmacokinetics?
A: Co-administration with drugs that are CYP1A2 inhibitors, such as ciprofloxacin, can significantly increase the systemic availability of ropinirole. [] On the other hand, co-administration with theophylline, a CYP1A2 substrate, did not significantly alter ropinirole pharmacokinetics. []
Q10: Does the use of estrogen replacement therapy affect ropinirole pharmacokinetics in women?
A: Yes, in women receiving long-term estrogen replacement therapy, oral clearance of ropinirole is reduced, and its elimination half-life is prolonged. [] Dosage adjustments may be necessary when starting or stopping estrogen replacement therapy during ropinirole treatment.
Q11: What preclinical models have been used to study the effects of ropinirole?
A: Preclinical studies have utilized various animal models, including rats with unilateral 6-hydroxydopamine lesions, to investigate the effects of ropinirole on motor symptoms, dyskinesia, and gliovascular changes associated with Parkinson's disease. []
Q12: What clinical trials have been conducted on ropinirole?
A: Numerous clinical trials have investigated the efficacy and safety of ropinirole in treating Parkinson's disease and restless legs syndrome (RLS). These trials include randomized, double-blind, placebo-controlled studies, crossover studies, and long-term efficacy studies. [, , , , , , , , , ]
Q13: Has ropinirole demonstrated efficacy in treating restless legs syndrome (RLS)?
A: Yes, clinical trials have demonstrated that ropinirole effectively reduces RLS symptoms, including the urge to move legs, uncomfortable sensations, and sleep disturbances. [, , , ]
Q14: What is the efficacy of ropinirole compared to other dopamine agonists in treating Parkinson's disease?
A: Clinical trials have compared ropinirole with other dopamine agonists like bromocriptine and pramipexole. [, , ] Results suggest that ropinirole exhibits comparable efficacy in improving motor symptoms, but individual patient responses may vary.
Q15: Are there any known mechanisms of resistance to ropinirole?
A: While ropinirole resistance is not extensively discussed in the provided articles, long-term use of dopamine agonists, in general, can lead to a decline in efficacy and the development of motor complications. [, , ] The exact mechanisms underlying this resistance are complex and require further investigation.
Q16: Does ropinirole induce or inhibit drug-metabolizing enzymes?
A: While the provided articles do not extensively discuss enzyme induction or inhibition by ropinirole, its metabolism by CYP1A2 suggests the potential for drug interactions. [, ] Co-administration with CYP1A2 inhibitors or inducers may necessitate dosage adjustments.
Q17: What are the alternative treatments for Parkinson's disease and RLS?
A: Alternative treatments for Parkinson's disease include levodopa (often considered the gold standard), other dopamine agonists (e.g., pramipexole, rotigotine), MAO-B inhibitors (e.g., selegiline, rasagiline), and COMT inhibitors (e.g., entacapone). [, , , , , , ] For RLS, alternatives include gabapentin, opioids, benzodiazepines, and iron supplementation. []
Q18: When was ropinirole first introduced as a treatment for Parkinson’s disease?
A: Ropinirole received approval for the treatment of Parkinson’s disease in the late 1990s. [, ]
Q19: Can ropinirole be used as monotherapy in early Parkinson’s disease?
A: Yes, ropinirole is approved for use as both early monotherapy and as an adjunct to levodopa in the treatment of Parkinson's disease. [, , , , ]
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
![Azuleno[6,5-b]furan-2,6(3H,4H)-dione, 3a,7,7a,8,9,9a-hexahydro-4-hydroxy-3,5,8-trimethyl-, [3R-(3alpha,3aalpha,4alpha,7abeta,8beta,9aalpha)]-](/img/structure/B1195771.png)
![2-hydroxy-2-[(8S,9S,10R,13S,14S)-11-hydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-ylidene]acetaldehyde](/img/structure/B1195773.png)