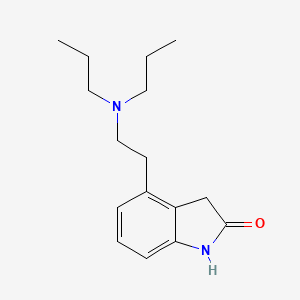
Ropinirole
Übersicht
Beschreibung
Ropinirol ist ein nicht-ergoliner Dopaminagonist, der hauptsächlich zur Behandlung der Parkinson-Krankheit und des Restless-Legs-Syndroms eingesetzt wird. Es wurde erstmals 1997 in den Vereinigten Staaten für den medizinischen Gebrauch zugelassen. Ropinirol wirkt, indem es Dopamin-D2-Rezeptoren im Gehirn stimuliert, was zur Linderung der Symptome im Zusammenhang mit Dopaminmangel beiträgt .
Herstellungsmethoden
Synthesewege und Reaktionsbedingungen
Ropinirol kann durch verschiedene Methoden synthetisiert werden. Eine gängige Methode beinhaltet die Reduktion eines 2-Nitrophenylessigsäure-Vorläufers, gefolgt von spontaner Cyclisierung. Eine andere Methode beinhaltet die reduktive Cyclisierung von 2-(2'-Brom-ethyl)-β-Nitrostyrol unter Verwendung von Palladium auf Kohlenstoff (Pd/C) als Katalysator .
Industrielle Produktionsmethoden
In industriellen Umgebungen wird Ropinirolhydrochlorid häufig durch Lösen der Verbindung in Lösungsmitteln wie Ethanol, Methanol oder Ethylacetat hergestellt, gefolgt von der Zugabe von Pd/C. Das Gemisch wird dann umgesetzt, um das gewünschte Produkt zu erhalten .
Wirkmechanismus
Ropinirole is a non-ergoline dopamine agonist primarily used to treat the symptoms of Parkinson’s disease and Restless Legs Syndrome . This article will delve into the mechanism of action of this compound, covering its primary targets, mode of action, affected biochemical pathways, pharmacokinetics, results of action, and the influence of environmental factors on its action.
Target of Action
This compound primarily targets the D2 and D3 dopamine receptor subtypes . It has a high relative in vitro specificity and full intrinsic activity at these receptors . This compound binds with higher affinity to D3 receptors, which are concentrated in the limbic areas of the brain and may be responsible for some of the neuropsychiatric effects .
Mode of Action
The exact mechanism of action of this compound as a treatment for Parkinson’s disease is unknown. It is believed to be related to its ability to selectively stimulate dopamine d2 receptors within the caudate-putamen system in the brain . This stimulation is thought to result in increased dopaminergic activity, which is typically deficient in conditions like Parkinson’s disease.
Biochemical Pathways
This compound’s interaction with D2 and D3 receptors influences several biochemical pathways. For instance, it has been found to interact with proteins in the Parkinson’s disease network, the retrograde endocannabinoid signaling pathway, and the neuronal nitric oxide synthase signaling network . These interactions can lead to various downstream effects, potentially influencing the symptoms and progression of diseases like Parkinson’s.
Pharmacokinetics
This compound is rapidly and almost completely absorbed when taken as oral tablets, and it is extensively distributed from the vascular compartment . The bioavailability is approximately 50% . This compound is metabolized in the liver, primarily by the cytochrome P450 (CYP) isoenzyme CYP1A2, to inactive metabolites . It is eliminated with a half-life of approximately 6 hours . Clearance is slower for patients older than 65 years compared with those who are younger, and in women taking hormone replacement therapy compared with those who are not .
Result of Action
The stimulation of dopamine D2 receptors by this compound in the brain is believed to increase dopaminergic activity, which can help alleviate the symptoms of Parkinson’s disease and Restless Legs Syndrome . .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, the presence of other medications can affect its action. The CYP1A2 inhibitor ciprofloxacin produced increases in the plasma concentrations of this compound when these two drugs were coadministered . Additionally, lifestyle factors such as cigarette smoking can also influence the action of this compound, as CYP1A2 is known to be induced by smoking .
Wissenschaftliche Forschungsanwendungen
Ropinirol hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Es wird als Modellverbindung in Studien verwendet, die Dopaminagonisten betreffen.
Biologie: Ropinirol wird verwendet, um die Auswirkungen von Dopaminagonisten auf zelluläre Signalwege zu untersuchen.
Medizin: Es wird häufig zur Behandlung der Parkinson-Krankheit und des Restless-Legs-Syndroms eingesetzt.
Wirkmechanismus
Ropinirol übt seine Wirkung aus, indem es selektiv Dopamin-D2-Rezeptoren innerhalb des Striatum-Systems im Gehirn stimuliert. Diese Stimulation trägt dazu bei, den Dopaminmangel auszugleichen, der bei Erkrankungen wie der Parkinson-Krankheit beobachtet wird. Ropinirol hat eine hohe Affinität für D2- und D3-Rezeptoren, wobei es eine höhere Affinität für D3-Rezeptoren aufweist .
Biochemische Analyse
Biochemical Properties
Ropinirole plays a crucial role in biochemical reactions by acting as a dopamine receptor agonist. It specifically targets dopamine D2 and D3 receptors, with a higher affinity for D3 receptors . By binding to these receptors, this compound stimulates dopaminergic activity in the brain, which helps alleviate symptoms of Parkinson’s disease and Restless Legs Syndrome . The interaction with these receptors is essential for its therapeutic effects.
Cellular Effects
This compound influences various cellular processes, particularly in neurons. It enhances dopaminergic signaling, which is vital for motor control and coordination . This compound also affects gene expression by modulating the activity of dopamine receptors, leading to changes in cellular metabolism and function . Additionally, this compound has been shown to protect neurons from apoptosis and reduce inflammation in certain cellular models .
Molecular Mechanism
At the molecular level, this compound exerts its effects by binding to dopamine D2 and D3 receptors, which are G-protein-coupled receptors . This binding activates intracellular signaling pathways, including the inhibition of adenylate cyclase and the modulation of ion channels . These actions result in increased dopaminergic activity, which helps improve motor function and reduce symptoms of Parkinson’s disease and Restless Legs Syndrome .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound have been observed to change over time. Studies have shown that this compound is stable under standard conditions but can degrade under extreme conditions . Long-term exposure to this compound has been associated with sustained improvements in motor function and reduced “off-time” in patients with Parkinson’s disease . Prolonged use can also lead to tolerance and the need for higher doses to achieve the same therapeutic effects .
Dosage Effects in Animal Models
In animal models, the effects of this compound vary with different dosages. Low doses of this compound have been shown to improve motor function and reduce symptoms of Parkinson’s disease . High doses can lead to adverse effects such as dyskinesia and compulsive behaviors . Studies have also indicated a threshold effect, where doses above a certain level do not provide additional therapeutic benefits and may increase the risk of side effects .
Metabolic Pathways
This compound is metabolized primarily in the liver by the cytochrome P450 enzyme CYP1A2 . The major metabolic pathways include N-despropylation and hydroxylation, resulting in the formation of inactive metabolites . These metabolites are then excreted through the kidneys. The metabolism of this compound can be influenced by other drugs that affect CYP1A2 activity, leading to potential drug interactions .
Transport and Distribution
This compound is widely distributed throughout the body, with a volume of distribution of approximately 7.5 L/kg . It is about 40% bound to plasma proteins and has a blood-to-plasma ratio of 1:1 . The compound is rapidly absorbed and extensively distributed from the vascular compartment to various tissues . The transport and distribution of this compound are crucial for its therapeutic effects, as it needs to reach the brain to exert its dopaminergic activity .
Subcellular Localization
The subcellular localization of this compound is primarily within the cytoplasm and at the cell membrane, where it interacts with dopamine receptors . The compound’s localization is influenced by its lipophilicity and the presence of specific transporters that facilitate its entry into cells . This compound’s activity is dependent on its ability to reach and bind to dopamine receptors, which are predominantly located on the cell membrane .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions
Ropinirole can be synthesized through several methods. One common method involves the reduction of a 2-nitrophenyl acetic acid precursor followed by spontaneous cyclization. Another method involves the reductive cyclization of 2-(2’-bromoethyl) β-nitrostyrene using palladium on carbon (Pd/C) as a catalyst .
Industrial Production Methods
In industrial settings, this compound hydrochloride is often prepared by dissolving the compound in solvents such as ethanol, methanol, or ethyl acetate, followed by the addition of Pd/C. The mixture is then reacted to obtain the desired product .
Analyse Chemischer Reaktionen
Arten von Reaktionen
Ropinirol unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: Ropinirol kann oxidiert werden, um verschiedene Metaboliten zu bilden.
Reduktion: Die Reduktion von Nitrogruppen zu Aminen ist ein wichtiger Schritt bei seiner Synthese.
Substitution: Halogenierungs- und Sulfonierungsreaktionen sind ebenfalls an seiner Synthese beteiligt.
Häufige Reagenzien und Bedingungen
Oxidation: Häufige Oxidationsmittel sind Wasserstoffperoxid und Kaliumpermanganat.
Reduktion: Katalysatoren wie Palladium auf Kohlenstoff (Pd/C) werden für die reduktive Cyclisierung verwendet.
Substitution: Reagenzien wie p-Methylbenzolsulfonylchlorid und Pyridin werden in Substitutionsreaktionen verwendet.
Hauptprodukte, die gebildet werden
Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, umfassen verschiedene Zwischenprodukte, die schließlich zur Bildung von Ropinirolhydrochlorid führen .
Vergleich Mit ähnlichen Verbindungen
Ähnliche Verbindungen
Pramipexol: Ein weiterer nicht-ergoliner Dopaminagonist, der zur Behandlung der Parkinson-Krankheit und des Restless-Legs-Syndroms eingesetzt wird.
Einzigartigkeit
Ropinirol ist einzigartig in seiner hohen Affinität für D3-Rezeptoren im Vergleich zu anderen Dopaminagonisten. Diese Spezifität könnte zu seiner Wirksamkeit bei der Behandlung von Symptomen der Parkinson-Krankheit und des Restless-Legs-Syndroms beitragen .
Eigenschaften
IUPAC Name |
4-[2-(dipropylamino)ethyl]-1,3-dihydroindol-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H24N2O/c1-3-9-18(10-4-2)11-8-13-6-5-7-15-14(13)12-16(19)17-15/h5-7H,3-4,8-12H2,1-2H3,(H,17,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UHSKFQJFRQCDBE-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCN(CCC)CCC1=C2CC(=O)NC2=CC=C1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H24N2O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
91374-20-8 (hydrochloride) | |
| Record name | Ropinirole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0091374219 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID8045195 | |
| Record name | Ropinirole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8045195 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
260.37 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
410.5±45.0 °C at 760 mmHg | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Solubility |
3.53e-01 g/L | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Ropinirole is a non-ergoline dopamine agonist. Ropinirole has the highest affinity at the D3 receptors, which are concentrated in the limbic areas of the brain and may be responsible for some of the neuropsychiatric effects. The exact mechanism of action of ropinirole as a treatment for Parkinson’s disease is unknown, however, it is believed to be related to its ability to selectively stimulate dopamine D2 receptors within the caudate-putamen system in the brain. This system affects body movement. Negligible affinity is seen for ropinirole at α2 adrenoreceptors in the periphery and 5HT-1 receptor. Ropinirole has no affinity at the D1-like receptors, benzodiazepine or GABA receptors. The precise mechanism of action of ropinirole as a treatment for Restless Legs Syndrome is unknown, however, it is believed to be related to its ability to stimulate dopamine receptors., The present study determined its affinity and agonist efficacy at recombinant human (h) dopamine hD2, hD3 and hD4 and serotonin (5-HT) h5-HT1A, h5-HT1B and h5-HT1D receptors. Roxindole exhibited high affinity at hD3 as well as at hD2 (short isoform) and hD4 (4-repeat isoform) receptors (pKi values 8.93, 8.55 and 8.23, respectively). Further, it displayed high affinity at hS-HT1A receptors (pKi = 9.42) but modest affinity at 5-HT1B and 5-HT1D receptors (pKi values 6.00 and 7.05, respectively). In [35S]GTPgammaS binding experiments, roxindole was >20-fold more potent in stimulating [35S]GTPgammaS binding at hD3 than at hD2 or hD4 receptors (pEC50 = 9.23 vs. 7.88 and 7.69). However, whereas roxindole exhibited partial agonist activity at hD3 and hD4 sites (Emax = 30.0% and 35.1%, respectively, relative to dopamine = 100%), it only weakly activated hD2 receptors (Emax = 10.5%). Roxindole potently blocked dopamine-stimulated [35S]GTPgammaS binding at hD2 receptors (pkappaB = 9.05). In comparison, the dopamine receptor agonist, (-)quinpirole, acted as a partial agonist at hD3 and hD4 sites (Emax = 67.4% and 66.3%, respectively) but surpassed the efficacy of dopamine at hD2 receptors (Emax = 132%). At h5-HT1A receptors, roxindole behaved as a high affinity (pKi = 9.42) partial agonist (Emax = 59.6%, relative to 5-HT = 100%), whereas (-)quinpirole had negligible activity. The selective 5-HT1A antagonist, WAY 100,635, blocked roxindole (100 nM)-stimulated [35S]GTPgammaS binding at h5-HT1A receptors in a concentration-dependent manner (pkappaB = 9.28). Roxindole only weakly stimulated [35S]GTPgammaS binding at 5-HT1B and 5-HT1D receptors (Emax = 27.1% and 13.7%). The present data suggest that roxindole activates mainly D3 vs. D2 or D4 receptors and 5-HT1A vs. 5-HT1B or 5-HT1D receptors. Activation of D3 and/or 5-HT1A receptors may thus contribute to its potential antidepressant properties., The aim of the present study was to characterize functional responses to ropinirole, its major metabolites in man (SKF-104557 (4-[2-(propylamino)ethyl]-2-(3H) indolone), SKF-97930 (4-carboxy-2-(3H) indolone)) and other dopamine receptor agonists at human dopamine D2(long) (hD2), D3 (hD3) and D4.4 (hD4) receptors separately expressed in Chinese hamster ovary cells using microphysiometry. 2. All the receptor agonists tested (ropinirole, SKF-104557, SKF-97930, bromocriptine, lisuride, pergolide, pramipexole, talipexole, dopamine) increased extracellular acidification rate in Chinese hamster ovary clones expressing the human D2, D3 or D4 receptor. The pEC50s of ropinirole at hD2, hD3 and hD4 receptors were 7.4, 8.4 and 6.8, respectively. Ropinirole is therefore at least 10 fold selective for the human dopamine D3 receptor over the other D2 receptor family members. 3. At the hD2 and hD3 dopamine receptors all the compounds tested were full agonists as compared to quinpirole. Talipexole and the ropinirole metabolite, SKF-104557, were partial agonists at the hD4 receptor. 4. Bromocriptine and lisuride had a slow onset of agonist action which precluded determination of EC50s. 5. The rank order of agonist potencies was dissimilar to the rank order of radioligand binding affinities at each of the dopamine receptor subtypes. Functional selectivities of the dopamine receptor agonists, as measured in the microphysiometer, were less than radioligand binding selectivities. 6. The results show that ropinirole is a full agonist at human D2, D3 and D4 dopamine receptors. SKF-104557 the major human metabolite of ropinirole, had similar radioligand binding affinities to, but lower functional potencies than, the parent compound., Ropinirole, which is a non-ergot dopamine agonist derivative, exerts therapeutic benefits in Parkinson's disease (PD). Based on recent studies implicating dopamine receptors 2 and 3 (D2R and D3R) as possible targets of ropinirole, we over-expressed these dopamine receptor genes in the dopamine-denervated striatum of rodents to reveal whether their over-expression modulated ropinirole activity. Adult Sprague-Dawley rats initially received unilateral 6-hydroxydopamine lesion of the medial forebrain bundle. At 1 month after surgery, successfully lesioned animals (3 or less forelimb akinesia score, and 8 or more apomorphine-induced rotations/min over 1 hr) were randomly assigned to intrastriatal injection (ipsilateral to the lesion) of blank lentiviral vector, D2R, D3R or both genes. At about 5 months post-lesion, ropinirole (0.2 mg/kg, i.p.) was administered daily for 9 consecutive days. The subtherapeutic dose of ropinirole improved the use of previously akinetic forelimb and produced robust circling behavior in lesioned animals with striatal over-expression of both D2R and D3R compared to lesioned animals that received blank vector. In contrast, the subtherapeutic dose of ropinirole generated only modest motor effects in lesioned animals with sole over-expression of D2R or D3R. Western immunoblot and autoradiographic assays showed enhanced D2R and D3R protein levels coupled with normalized D2R and D3R binding in the ventral striatum of lesioned animals with lentiviral over-expression of both D2R and D3R relative to vehicle-treated lesioned animals. Immunohistochemical analyses showed that D2R and D3R GFP fluorescent cells colocalized with enkephalin and substance P immunoreactive medium spiny neurons. These data support the use of the subtherapeutic dose of ropinirole in a chronic model of PD., Ropinirole hydrochloride, a dipropylaminoethyl indolone derivative, is a nonergot-derivative dopamine receptor agonist. In in vitro binding studies, ropinirole demonstrated high binding specificity for and intrinsic activity at dopamine D2 receptors compared with other dopamine receptor agonists (e.g., bromocriptine, pergolide), having a higher affinity for the D3 subtype than for the D2 or D4 subtypes. Ropinirole binds with moderate affinity to opiate receptors but has little or no affinity for alpha1-, alpha2-, or beta-adrenergic; dopamine D1; benzodiazepine; gamma-aminobutyric acid (GABA); serotonin type 1 (5-hydroxytryptamine (5-HT1)); serotonin type 2 (5-HT2); or muscarinic receptors. | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ROPINIROLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
91374-21-9 | |
| Record name | Ropinirole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=91374-21-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ropinirole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0091374219 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ropinirole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758917 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Ropinirole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8045195 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2H-Indol-2-one, 4-[2-(dipropylamino)ethyl]-1,3-dihydro | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.110.353 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ROPINIROLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/030PYR8953 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | ROPINIROLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
243-250 °C, 243 - 250 °C | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does ropinirole interact with its target?
A: this compound is a non-ergoline dopamine agonist that primarily targets dopamine D2-like receptors, exhibiting a high affinity for both D2 and D3 subtypes. [, , , ] It mimics the action of dopamine by binding to these receptors, leading to downstream signaling events.
Q2: What are the downstream effects of this compound binding to dopamine receptors?
A: The binding of this compound to D2-like receptors, particularly in the striatum, helps restore dopaminergic signaling, which is deficient in Parkinson’s disease. [, , ] This, in turn, helps alleviate motor symptoms such as tremors, rigidity, and bradykinesia.
Q3: What is the molecular formula and weight of this compound?
A3: this compound has the molecular formula C16H24N2O2 and a molecular weight of 276.37 g/mol.
Q4: Is there any spectroscopic data available for this compound?
A: While the provided research articles do not delve into detailed spectroscopic data, studies often employ techniques like UV spectrophotometry and RP-HPLC for this compound analysis. []
Q5: What are the different formulations of this compound available?
A: this compound is available in both immediate-release and 24-hour prolonged-release formulations. [, , , , , ] The prolonged-release formulation aims to maintain more consistent dopaminergic activity with steadier plasma levels, potentially leading to improved tolerability and compliance.
Q6: How is this compound absorbed and distributed in the body?
A: this compound is rapidly and almost completely absorbed after oral administration. [, , ] It exhibits good distribution throughout the body, crossing the blood-brain barrier to reach its target sites in the brain. []
Q7: What is the primary route of this compound metabolism?
A: this compound is extensively metabolized in the liver, primarily by the cytochrome P450 (CYP) isoenzyme CYP1A2. [, , ] It also undergoes metabolism by CYP3A4/5 to a lesser extent. []
Q8: How is this compound eliminated from the body?
A: The primary route of this compound elimination is renal excretion, with approximately 60-90% of the administered dose being eliminated through urine. []
Q9: Does the presence of other drugs affect this compound pharmacokinetics?
A: Co-administration with drugs that are CYP1A2 inhibitors, such as ciprofloxacin, can significantly increase the systemic availability of this compound. [] On the other hand, co-administration with theophylline, a CYP1A2 substrate, did not significantly alter this compound pharmacokinetics. []
Q10: Does the use of estrogen replacement therapy affect this compound pharmacokinetics in women?
A: Yes, in women receiving long-term estrogen replacement therapy, oral clearance of this compound is reduced, and its elimination half-life is prolonged. [] Dosage adjustments may be necessary when starting or stopping estrogen replacement therapy during this compound treatment.
Q11: What preclinical models have been used to study the effects of this compound?
A: Preclinical studies have utilized various animal models, including rats with unilateral 6-hydroxydopamine lesions, to investigate the effects of this compound on motor symptoms, dyskinesia, and gliovascular changes associated with Parkinson's disease. []
Q12: What clinical trials have been conducted on this compound?
A: Numerous clinical trials have investigated the efficacy and safety of this compound in treating Parkinson's disease and restless legs syndrome (RLS). These trials include randomized, double-blind, placebo-controlled studies, crossover studies, and long-term efficacy studies. [, , , , , , , , , ]
Q13: Has this compound demonstrated efficacy in treating restless legs syndrome (RLS)?
A: Yes, clinical trials have demonstrated that this compound effectively reduces RLS symptoms, including the urge to move legs, uncomfortable sensations, and sleep disturbances. [, , , ]
Q14: What is the efficacy of this compound compared to other dopamine agonists in treating Parkinson's disease?
A: Clinical trials have compared this compound with other dopamine agonists like bromocriptine and pramipexole. [, , ] Results suggest that this compound exhibits comparable efficacy in improving motor symptoms, but individual patient responses may vary.
Q15: Are there any known mechanisms of resistance to this compound?
A: While this compound resistance is not extensively discussed in the provided articles, long-term use of dopamine agonists, in general, can lead to a decline in efficacy and the development of motor complications. [, , ] The exact mechanisms underlying this resistance are complex and require further investigation.
Q16: Does this compound induce or inhibit drug-metabolizing enzymes?
A: While the provided articles do not extensively discuss enzyme induction or inhibition by this compound, its metabolism by CYP1A2 suggests the potential for drug interactions. [, ] Co-administration with CYP1A2 inhibitors or inducers may necessitate dosage adjustments.
Q17: What are the alternative treatments for Parkinson's disease and RLS?
A: Alternative treatments for Parkinson's disease include levodopa (often considered the gold standard), other dopamine agonists (e.g., pramipexole, rotigotine), MAO-B inhibitors (e.g., selegiline, rasagiline), and COMT inhibitors (e.g., entacapone). [, , , , , , ] For RLS, alternatives include gabapentin, opioids, benzodiazepines, and iron supplementation. []
Q18: When was this compound first introduced as a treatment for Parkinson’s disease?
A: this compound received approval for the treatment of Parkinson’s disease in the late 1990s. [, ]
Q19: Can this compound be used as monotherapy in early Parkinson’s disease?
A: Yes, this compound is approved for use as both early monotherapy and as an adjunct to levodopa in the treatment of Parkinson's disease. [, , , , ]
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.
![N-[1-[4-(4-methylphenyl)-2-thiazolyl]-4-piperidinyl]-2,3-dihydro-1,4-benzodioxin-6-sulfonamide](/img/structure/B1195756.png)
![Azuleno[6,5-b]furan-2,6(3H,4H)-dione, 3a,7,7a,8,9,9a-hexahydro-4-hydroxy-3,5,8-trimethyl-, [3R-(3alpha,3aalpha,4alpha,7abeta,8beta,9aalpha)]-](/img/structure/B1195771.png)