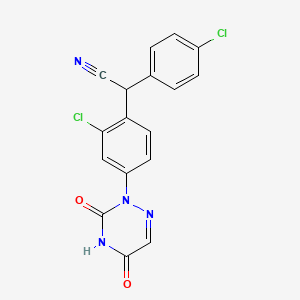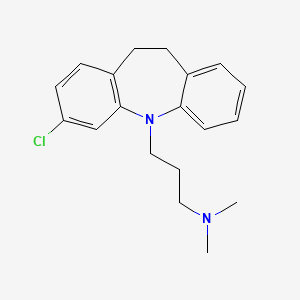
クロミプラミン
概要
説明
クロミプラミンは、主に強迫性障害の治療に使用される三環系抗うつ薬です。また、主要なうつ病、パニック障害、慢性疼痛などの他の病状の治療にも有効です。 クロミプラミンは、1964年にスイスの製薬会社チバガイギーによって発見され、アナフラニルなどのブランド名で販売されています .
2. 製法
合成経路と反応条件: クロミプラミンは、イミノジベンジルを出発物質とする多段階プロセスによって合成されます。主な工程は以下の通りです。
塩素化: イミノジベンジルを塩素化して3-クロロイミノジベンジルを生成します。
還元: 塩素化生成物を還元して3-クロロ-10,11-ジヒドロ-5H-ジベンゾ[b,f]アゼピンを生成します。
工業生産方法: クロミプラミンの工業生産は、通常、同じ合成経路に従いますが、より大規模です。このプロセスには、高純度と高収率を確保するために、反応条件を厳密に制御することが含まれます。 最終生成物は、多くの場合、安定性と製剤の容易さのために、塩酸塩の形に変換されます .
作用機序
クロミプラミンは、中枢神経系におけるセロトニンとノルエピネフリンの再取り込みを阻害することによって作用します。これにより、シナプス間隙におけるこれらの神経伝達物質の濃度が上昇し、神経伝達の強化につながります。 この化合物は、ヒスタミン-H1受容体、α1アドレナリン受容体、およびムスカリン受容体も遮断するため、鎮静作用、降圧作用、抗コリン作用に寄与します .
類似化合物:
イミプラミン: 同様の適応症で使用される別の三環系抗うつ薬です。
アミトリプチリン: 鎮静作用で知られており、うつ病と慢性疼痛の治療に使用されます。
ノルトリプチリン: 異なる副作用プロファイルを持つ二次アミン三環系抗うつ薬.
クロミプラミンの独自性: クロミプラミンは、他の三環系抗うつ薬と比較して、セロトニン再取り込みを強力に阻害するという点でユニークです。これは、強迫性障害の治療に特に効果的です。 さらに、その代謝物である脱メチルクロミプラミンは、ノルエピネフリン再取り込みを選択的に阻害するため、二重の作用機序を提供します .
科学的研究の応用
Clomipramine has a wide range of scientific research applications:
Chemistry: Used as a model compound in the study of tricyclic antidepressants.
Biology: Investigated for its effects on neurotransmitter systems and neuronal autophagy.
Industry: Utilized in the development of diagnostic tools and eco-friendly sensors.
準備方法
Synthetic Routes and Reaction Conditions: Clomipramine is synthesized through a multi-step process starting from iminodibenzyl. The key steps involve:
Chlorination: Iminodibenzyl is chlorinated to form 3-chloroiminodibenzyl.
Reduction: The chlorinated product is reduced to 3-chloro-10,11-dihydro-5H-dibenzo[b,f]azepine.
Alkylation: The final step involves alkylation with 3-dimethylaminopropyl chloride to yield clomipramine.
Industrial Production Methods: Industrial production of clomipramine typically follows the same synthetic route but on a larger scale. The process involves stringent control of reaction conditions to ensure high purity and yield. The final product is often converted to its hydrochloride salt form for stability and ease of formulation .
化学反応の分析
反応の種類: クロミプラミンは、以下を含むいくつかの種類の化学反応を起こします。
酸化: クロミプラミンは、N-オキシド誘導体に変換されて酸化される可能性があります。
還元: この化合物は、その脱メチル代謝物、脱メチルクロミプラミンに還元することができます。
一般的な試薬と条件:
酸化: 過酸化水素または過酸が一般的に使用されます。
還元: 水素化リチウムアルミニウムまたは水素化ホウ素ナトリウムが一般的な還元剤です。
生成される主要な生成物:
脱メチルクロミプラミン: 還元によって生成されます。
N-オキシド誘導体: 酸化によって生成されます.
4. 科学研究への応用
クロミプラミンは、幅広い科学研究への応用があります。
化学: 三環系抗うつ薬の研究におけるモデル化合物として使用されます。
生物学: 神経伝達物質系とニューロンオートファジーへの影響について調査されています.
類似化合物との比較
Imipramine: Another tricyclic antidepressant used for similar indications.
Amitriptyline: Known for its sedative properties and used in the treatment of depression and chronic pain.
Nortriptyline: A secondary amine tricyclic antidepressant with a different side effect profile.
Uniqueness of Clomipramine: Clomipramine is unique due to its strong inhibition of serotonin reuptake compared to other tricyclic antidepressants. This makes it particularly effective in treating obsessive-compulsive disorder. Additionally, its metabolite, desmethylclomipramine, preferentially inhibits norepinephrine reuptake, providing a dual mechanism of action .
特性
IUPAC Name |
3-(2-chloro-5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GDLIGKIOYRNHDA-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCCN1C2=CC=CC=C2CCC3=C1C=C(C=C3)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H23ClN2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
17321-77-6 (mono-hydrochloride) | |
| Record name | Clomipramine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000303491 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID6022844 | |
| Record name | Clomipramine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022844 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
314.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Clomipramine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015372 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
160-170 °C at 3.00E-01 mm Hg, 160-170 °C at 0.3 mm Hg | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clomipramine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7746 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
1.44e-02 g/L | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clomipramine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015372 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Clomipramine is a strong, but not completely selective serotonin reuptake inhibitor (SRI), as the active main metabolite desmethyclomipramine acts preferably as an inhibitor of noradrenaline reuptake. α1-receptor blockage and β-down-regulation have been noted and most likely play a role in the short term effects of clomipramine. A blockade of sodium-channels and NDMA-receptors might, as with other tricyclics, account for its effect in chronic pain, in particular the neuropathic type., The pharmacology of clomipramine is complex and in many ways resembles that of other antidepressants, particularly those agents (eg, selective serotonin-reuptake inhibitors, trazodone) that predominantly potentiate the pharmacologic effects of serotonin (5-HT). Although clomipramine's principal pharmacologic effect in vitro is the selective inhibition of serotonin reuptake, in vivo the drug's pharmacologic activity is not so selective because of the action of its demethylated metabolite, desmethylclomipramine, as an inhibitor of norepinephrine reuptake. As a result of this and other effects, clomipramine also shares the pharmacologic profile of other tricyclic antidepressants., The precise mechanism of action that is responsible for the efficacy of clomipramine in the treatment of obsessive-compulsive disorder is unclear. However, because of its pronounced potency in blocking serotonin reuptake at the presynaptic neuronal membrane and its efficacy in the treatment of obsessive-compulsive disorder, a serotonin hypothesis has been developed to explain the pathogenesis of the condition. The hypothesis postulates that a dysregulation of serotonin is responsible for obsessive-compulsive disorder and that clomipramine is effective because it corrects this imbalance., Clomipramine and its principal metabolite, desmethylclomipramine, have been shown to block the reuptake of serotonin and norepinephrine, respectively, at the presynaptic neuronal membrane. The effects of serotonin and norepinephrine may thus be potentiated. However, it has been suggested that postsynaptic receptor modification is mainly responsible for the antidepressant action observed during long-term administration of antidepressant agents. During long-term therapy with most antidepressants (eg, tricyclic antidepressants, monoamine oxidase [MAO] inhibitors), these adaptive changes generally consist of subsensitivity of the noradrenergic adenylate cyclase system in association with a decrease in the number of beta-adrenergic receptors; such effects on noradrenergic receptor function commonly are referred to as "down-regulation." In addition, some antidepressants reportedly decrease the number of 5-HT binding sites following chronic administration., Clomipramine's principal metabolite, desmethylclomipramine, is an inhibitor of norepinephrine reuptake. Clomipramine decreases the concentration of 3-methoxy-4-hydroxyphenylglycol (MHPG), a metabolite of norepinephrine, in CSF in patients with obsessive-compulsive disorder. Patients with depressive affective (mood) disorders (e.g., major depressive episode) also exhibit decreases in concentrations of 5-HIAA and MHPG in CSF during treatment with clomipramine. The decrease in the concentration of 5-HIAA in CSF was correlated with inhibition of the in vitro uptake of 3H-serotonin in plasma. The change in concentration of MHPG in CSF during clomipramine therapy was correlated with amelioration of depression., For more Mechanism of Action (Complete) data for Clomipramine (10 total), please visit the HSDB record page. | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clomipramine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7746 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
N-[3-(3-chloro-10,11-dihydro-5H-dibenzo[b,f]azepin-t-yl)propyl]-N,N',N'-trimethylpropane-1,3-diamine, 3-(3-chloro-5H-dibenzo[b,f]azepin-5-yl]-N,N-dimethylpropan-1-amine, 3-(3,7-dichloro-10,11-dihydro-5H-dibenzol[b,f]azepin-5-yl)-N,N-dimethylpropan-1-amine, 3-chloro-5-[3-(dimethylamino)propyl]-10,11-dihydro-5H-dibenz[b,f]azepine, For more Impurities (Complete) data for Clomipramine (11 total), please visit the HSDB record page. | |
| Record name | Clomipramine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7746 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
303-49-1 | |
| Record name | Clomipramine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=303-49-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clomipramine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000303491 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | clomipramine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=169865 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Clomipramine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022844 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clomipramine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.005.587 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLOMIPRAMINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/NUV44L116D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Clomipramine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7746 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Clomipramine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015372 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
191.5-192, 189.5 °C | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clomipramine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015372 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![[4-[[(2S)-6-amino-2-[[(2S)-2-[[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[4-[[[4-[(2,5-dioxopyrrol-1-yl)methyl]cyclohexanecarbonyl]amino]methyl]triazol-1-yl]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethylamino]-2-oxoethoxy]acetyl]amino]-3-phenylpropanoyl]amino]hexanoyl]amino]phenyl]methyl [(19S)-10,19-diethyl-7-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaen-19-yl] carbonate](/img/structure/B1669145.png)