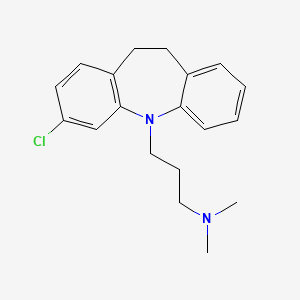
Clomipramin
Übersicht
Beschreibung
Clomipramin ist ein trizyklisches Antidepressivum, das in erster Linie zur Behandlung von Zwangsstörungen eingesetzt wird. Es ist auch wirksam bei der Behandlung anderer Erkrankungen wie Major Depression, Panikstörung und chronischen Schmerzen. This compound wurde 1964 vom Schweizer Pharmaunternehmen Ciba-Geigy entdeckt und wird unter anderem unter dem Markennamen Anafranil verkauft .
Wirkmechanismus
Target of Action
Clomipramine, a tricyclic antidepressant (TCA), primarily targets the serotonin and norepinephrine reuptake transporters . These transporters are responsible for the reabsorption of serotonin and norepinephrine into the presynaptic neuron after they have completed their function of transmitting a neural impulse. By inhibiting these transporters, clomipramine increases the levels of serotonin and norepinephrine in the synaptic cleft, enhancing their neurotransmission .
Mode of Action
Clomipramine acts as a potent inhibitor of serotonin reuptake and a less potent inhibitor of norepinephrine reuptake . It binds to the serotonin and norepinephrine transporters, blocking their action, which leads to an increased concentration of these neurotransmitters in the synaptic cleft . This results in prolonged neurotransmitter action on the post-synaptic receptors, enhancing serotonergic and noradrenergic neurotransmission .
Biochemical Pathways
The enhanced serotonergic and noradrenergic neurotransmission resulting from clomipramine’s action can affect various biochemical pathways. For instance, it has been reported that clomipramine can interfere with the autophagic flux, a crucial cellular degradation pathway . This interference can lead to changes in the metabolomic pathways associated with the pathophysiology of depression .
Pharmacokinetics
Clomipramine is rapidly absorbed from the gastrointestinal tract and is extensively metabolized in the liver, primarily by the CYP2D6 enzyme, to its active metabolite, desmethylclomipramine . The bioavailability of clomipramine is approximately 50% . It has a high protein binding capacity of 96-98% . The elimination half-life of clomipramine is 19-37 hours, and that of desmethylclomipramine is 54-77 hours . It is excreted in the urine (51-60%) and feces (24-32%) .
Result of Action
The increased levels of serotonin and norepinephrine in the synaptic cleft due to clomipramine’s action can lead to various molecular and cellular effects. These include down-regulation of cerebral cortical β-adrenergic receptors and sensitization of post-synaptic serotonergic receptors with chronic use . This can result in mood elevation in depressed individuals . Moreover, clomipramine has been found to inhibit neuronal autophagic flux, which can have significant implications for cellular viability and function .
Action Environment
Environmental factors can influence the action, efficacy, and stability of clomipramine. For instance, factors such as pollution and smoking can enhance the risk of negative outcomes . Furthermore, the route of administration can affect the drug’s action. For example, intranasal administration of clomipramine has been associated with more significant changes in the metabolites composition in the frontal cortex compared to other routes .
Wissenschaftliche Forschungsanwendungen
Clomipramin hat eine große Bandbreite an Anwendungen in der wissenschaftlichen Forschung:
Chemie: Als Modellverbindung in der Untersuchung von trizyklischen Antidepressiva eingesetzt.
Biologie: Untersucht wegen seiner Auswirkungen auf Neurotransmittersysteme und neuronale Autophagie.
Industrie: Wird bei der Entwicklung von Diagnosewerkzeugen und umweltfreundlichen Sensoren eingesetzt.
5. Wirkmechanismus
This compound wirkt, indem es die Wiederaufnahme von Serotonin und Noradrenalin im zentralen Nervensystem hemmt. Dies führt zu erhöhten Konzentrationen dieser Neurotransmitter im synaptischen Spalt, was zu einer verstärkten Neurotransmission führt. Die Verbindung blockiert auch Histamin-H1-Rezeptoren, α1-adrenerge Rezeptoren und muskarinische Rezeptoren, was zu ihren sedativen, blutdrucksenkenden und anticholinergen Wirkungen beiträgt .
Ähnliche Verbindungen:
Imipramin: Ein weiteres trizyklisches Antidepressivum, das für ähnliche Indikationen verwendet wird.
Amitriptylin: Bekannt für seine sedativen Eigenschaften und bei der Behandlung von Depressionen und chronischen Schmerzen eingesetzt.
Nortriptylin: Ein sekundäres Amin-trizyklisches Antidepressivum mit einem anderen Nebenwirkungsprofil.
Einzigartigkeit von this compound: this compound ist einzigartig aufgrund seiner starken Hemmung der Serotonin-Wiederaufnahme im Vergleich zu anderen trizyklischen Antidepressiva. Dies macht es besonders wirksam bei der Behandlung von Zwangsstörungen. Darüber hinaus hemmt sein Metabolit, Desmethylthis compound, bevorzugt die Noradrenalin-Wiederaufnahme, was einen dualen Wirkmechanismus bietet .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen: Clomipramin wird in einem mehrstufigen Verfahren aus Iminodibenzyl synthetisiert. Die wichtigsten Schritte umfassen:
Chlorierung: Iminodibenzyl wird chloriert, um 3-Chloriminodibenzyl zu bilden.
Reduktion: Das chlorierte Produkt wird zu 3-Chlor-10,11-Dihydro-5H-dibenzo[b,f]azepin reduziert.
Alkylierung: Der letzte Schritt beinhaltet die Alkylierung mit 3-Dimethylaminopropylchlorid, um this compound zu ergeben.
Industrielle Produktionsmethoden: Die industrielle Produktion von this compound erfolgt in der Regel nach dem gleichen Syntheseweg, jedoch im größeren Maßstab. Der Prozess beinhaltet eine strenge Kontrolle der Reaktionsbedingungen, um eine hohe Reinheit und Ausbeute zu gewährleisten. Das Endprodukt wird oft in seine Hydrochloridsalzform umgewandelt, um Stabilität und einfache Formulierung zu gewährleisten .
Analyse Chemischer Reaktionen
Reaktionstypen: Clomipramin durchläuft verschiedene Arten chemischer Reaktionen, darunter:
Oxidation: this compound kann oxidiert werden, um sein N-Oxid-Derivat zu bilden.
Reduktion: Die Verbindung kann zu ihrem Desmethylmetaboliten, Desmethylthis compound, reduziert werden.
Substitution: Halogenierungs- und Alkylierungsreaktionen können das Clomipraminmolekül modifizieren.
Häufige Reagenzien und Bedingungen:
Oxidation: Wasserstoffperoxid oder Persäuren werden üblicherweise verwendet.
Reduktion: Lithiumaluminiumhydrid oder Natriumborhydrid sind typische Reduktionsmittel.
Substitution: Halogenierungsmittel wie Chlor oder Brom und Alkylierungsmittel wie Alkylhalogenide.
Hauptprodukte, die gebildet werden:
Desmethylthis compound: Bildet sich durch Reduktion.
N-Oxid-Derivat: Bildet sich durch Oxidation.
Vergleich Mit ähnlichen Verbindungen
Imipramine: Another tricyclic antidepressant used for similar indications.
Amitriptyline: Known for its sedative properties and used in the treatment of depression and chronic pain.
Nortriptyline: A secondary amine tricyclic antidepressant with a different side effect profile.
Uniqueness of Clomipramine: Clomipramine is unique due to its strong inhibition of serotonin reuptake compared to other tricyclic antidepressants. This makes it particularly effective in treating obsessive-compulsive disorder. Additionally, its metabolite, desmethylclomipramine, preferentially inhibits norepinephrine reuptake, providing a dual mechanism of action .
Eigenschaften
IUPAC Name |
3-(2-chloro-5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GDLIGKIOYRNHDA-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCCN1C2=CC=CC=C2CCC3=C1C=C(C=C3)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H23ClN2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
17321-77-6 (mono-hydrochloride) | |
| Record name | Clomipramine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000303491 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID6022844 | |
| Record name | Clomipramine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022844 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
314.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Clomipramine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015372 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
160-170 °C at 3.00E-01 mm Hg, 160-170 °C at 0.3 mm Hg | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clomipramine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7746 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
1.44e-02 g/L | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clomipramine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015372 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Clomipramine is a strong, but not completely selective serotonin reuptake inhibitor (SRI), as the active main metabolite desmethyclomipramine acts preferably as an inhibitor of noradrenaline reuptake. α1-receptor blockage and β-down-regulation have been noted and most likely play a role in the short term effects of clomipramine. A blockade of sodium-channels and NDMA-receptors might, as with other tricyclics, account for its effect in chronic pain, in particular the neuropathic type., The pharmacology of clomipramine is complex and in many ways resembles that of other antidepressants, particularly those agents (eg, selective serotonin-reuptake inhibitors, trazodone) that predominantly potentiate the pharmacologic effects of serotonin (5-HT). Although clomipramine's principal pharmacologic effect in vitro is the selective inhibition of serotonin reuptake, in vivo the drug's pharmacologic activity is not so selective because of the action of its demethylated metabolite, desmethylclomipramine, as an inhibitor of norepinephrine reuptake. As a result of this and other effects, clomipramine also shares the pharmacologic profile of other tricyclic antidepressants., The precise mechanism of action that is responsible for the efficacy of clomipramine in the treatment of obsessive-compulsive disorder is unclear. However, because of its pronounced potency in blocking serotonin reuptake at the presynaptic neuronal membrane and its efficacy in the treatment of obsessive-compulsive disorder, a serotonin hypothesis has been developed to explain the pathogenesis of the condition. The hypothesis postulates that a dysregulation of serotonin is responsible for obsessive-compulsive disorder and that clomipramine is effective because it corrects this imbalance., Clomipramine and its principal metabolite, desmethylclomipramine, have been shown to block the reuptake of serotonin and norepinephrine, respectively, at the presynaptic neuronal membrane. The effects of serotonin and norepinephrine may thus be potentiated. However, it has been suggested that postsynaptic receptor modification is mainly responsible for the antidepressant action observed during long-term administration of antidepressant agents. During long-term therapy with most antidepressants (eg, tricyclic antidepressants, monoamine oxidase [MAO] inhibitors), these adaptive changes generally consist of subsensitivity of the noradrenergic adenylate cyclase system in association with a decrease in the number of beta-adrenergic receptors; such effects on noradrenergic receptor function commonly are referred to as "down-regulation." In addition, some antidepressants reportedly decrease the number of 5-HT binding sites following chronic administration., Clomipramine's principal metabolite, desmethylclomipramine, is an inhibitor of norepinephrine reuptake. Clomipramine decreases the concentration of 3-methoxy-4-hydroxyphenylglycol (MHPG), a metabolite of norepinephrine, in CSF in patients with obsessive-compulsive disorder. Patients with depressive affective (mood) disorders (e.g., major depressive episode) also exhibit decreases in concentrations of 5-HIAA and MHPG in CSF during treatment with clomipramine. The decrease in the concentration of 5-HIAA in CSF was correlated with inhibition of the in vitro uptake of 3H-serotonin in plasma. The change in concentration of MHPG in CSF during clomipramine therapy was correlated with amelioration of depression., For more Mechanism of Action (Complete) data for Clomipramine (10 total), please visit the HSDB record page. | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clomipramine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7746 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
N-[3-(3-chloro-10,11-dihydro-5H-dibenzo[b,f]azepin-t-yl)propyl]-N,N',N'-trimethylpropane-1,3-diamine, 3-(3-chloro-5H-dibenzo[b,f]azepin-5-yl]-N,N-dimethylpropan-1-amine, 3-(3,7-dichloro-10,11-dihydro-5H-dibenzol[b,f]azepin-5-yl)-N,N-dimethylpropan-1-amine, 3-chloro-5-[3-(dimethylamino)propyl]-10,11-dihydro-5H-dibenz[b,f]azepine, For more Impurities (Complete) data for Clomipramine (11 total), please visit the HSDB record page. | |
| Record name | Clomipramine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7746 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
303-49-1 | |
| Record name | Clomipramine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=303-49-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clomipramine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000303491 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | clomipramine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=169865 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Clomipramine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022844 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clomipramine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.005.587 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLOMIPRAMINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/NUV44L116D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Clomipramine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7746 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Clomipramine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015372 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
191.5-192, 189.5 °C | |
| Record name | Clomipramine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clomipramine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015372 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![1-(3,4-dichlorophenyl)-3-azabicyclo[3.1.0]hexane Hydrochloride](/img/structure/B1669140.png)