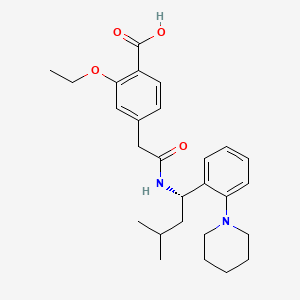
レパグリニド
概要
説明
科学的研究の応用
Repaglinide has several scientific research applications, including:
Chemistry: Used as a model compound for studying the synthesis and modification of antidiabetic drugs.
Biology: Studied for its effects on pancreatic β cells and insulin secretion.
Medicine: Used in clinical trials to evaluate its efficacy and safety in treating type 2 diabetes.
Industry: Employed in the development of new drug formulations and delivery systems to improve bioavailability and therapeutic outcomes
作用機序
レパグリニドは、膵臓のβ細胞にあるATP感受性カリウムチャネルに結合することで効果を発揮します。この結合はカリウムイオンの流出を阻害し、細胞膜の脱分極につながります。脱分極は電圧依存性カルシウムチャネルを開き、カルシウムイオンが細胞内に侵入することを可能にします。 カルシウムイオンの流入は、インスリン顆粒のエクソサイトーシスを引き起こし、インスリンの分泌が増加します . このメカニズムはグルコース依存性であり、インスリンの分泌はグルコースの存在下でのみ促進され、低血糖のリスクを軽減します .
類似の化合物との比較
レパグリニドは、多くの場合、次のものなどの他の糖尿病治療薬と比較されます。
ナテグリニド: 同様の作用機序を持つ別のメグリチニドですが、作用時間は短いです。
スルホニルウレア系薬: グリベンクラミドやグリメピリドなど。これらもインスリンの分泌を促進しますが、作用時間が長く、低血糖のリスクが高くなります。
メトホルミン: 肝臓でのグルコース産生を抑制し、インスリン感受性を改善するビグアナイド系薬ですが、インスリンの分泌を促進しません
レパグリニドは、その迅速な発現と短時間作用で、長期の低血糖を引き起こすことなく、食後の血糖値のコントロールに特に効果的です .
生化学分析
Biochemical Properties
Repaglinide interacts with specific proteins in the body, particularly in the pancreas. It binds to ATP-sensitive potassium channels on the surface of pancreatic beta cells . This binding inhibits potassium efflux, leading to depolarization of the cell membrane and subsequent insulin release .
Cellular Effects
Repaglinide has a profound effect on pancreatic beta cells. By stimulating insulin release, it helps regulate blood glucose levels. It also influences cell signaling pathways related to insulin secretion .
Molecular Mechanism
The molecular mechanism of Repaglinide involves its binding to ATP-sensitive potassium channels on pancreatic beta cells . This binding inhibits the efflux of potassium ions, causing the cell to depolarize. This depolarization triggers the opening of calcium channels, leading to an influx of calcium ions, which then stimulate the release of insulin .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Repaglinide are observed over time. It has been found to have a sustained release, ensuring safety and improving the efficacy of the drug . The drug’s stability and degradation over time are factors that are considered in its formulation .
Dosage Effects in Animal Models
The effects of Repaglinide in animal models vary with dosage. Studies have shown that it effectively lowers blood glucose levels in a dose-dependent manner .
Metabolic Pathways
Repaglinide is involved in metabolic pathways related to glucose regulation. It interacts with enzymes and cofactors in these pathways, influencing metabolic flux and metabolite levels .
Transport and Distribution
Repaglinide is transported and distributed within cells and tissues via specific transporters . Its localization and accumulation within cells can influence its efficacy .
Subcellular Localization
The subcellular localization of Repaglinide is primarily at the cell membrane of pancreatic beta cells, where it interacts with ATP-sensitive potassium channels . This localization is crucial for its function in stimulating insulin release .
準備方法
合成ルートと反応条件
レパグリニドは、いくつかの重要な中間体を用いた複数段階の工程を経て合成できます。 一般的な方法には、次の手順が含まれます :
エステル化: 3-ヒドロキシフェニル酢酸をエステル化してエチル3-ヒドロキシフェニルアセテートを生成します。
ホルミル化: 次に、エステルをホルミル化してエチル3-ホルミル-4-ヒドロキシフェニルアセテートを生成します。
酸化: ホルミル基をカルボン酸に酸化して、エチル3-カルボキシ-4-ヒドロキシフェニルアセテートを生成します。
エーテル化: ヒドロキシル基をエーテル化して、エチル3-カルボキシ-4-エトキシフェニルアセテートを生成します。
選択的加水分解: エステルを選択的に加水分解して、3-カルボキシ-4-エトキシフェニル酢酸を生成します。これは、レパグリニド合成における重要な中間体です。
工業的製造方法
レパグリニドの工業的製造には、収率と純度を最大限に高めるための反応条件の最適化が伴います。 これには、反応温度、時間、溶媒、基質比の制御が含まれます . このプロセスは、スケーラブルで環境に優しく、不純物が最小限に抑えられるように設計されています。
化学反応の分析
反応の種類
レパグリニドは、次のものなど、いくつかの種類の化学反応を起こします。
酸化: レパグリニドは酸化されて、さまざまな代謝物を生成する可能性があります。
還元: 還元反応は、レパグリニド分子の官能基を変換することができます。
置換: 置換反応は、芳香環や側鎖のさまざまな位置で起こることがあります。
一般的な試薬と条件
レパグリニドの合成と改変に使用される一般的な試薬には、次のものがあります。
酸化剤: 過マンガン酸カリウムや過酸化水素など。
還元剤: 水素化ホウ素ナトリウムや水素化リチウムアルミニウムなど。
置換試薬: ハロゲンやアルキル化剤など。
主な生成物
これらの反応から生成される主な生成物には、レパグリニドのさまざまな代謝物や誘導体が含まれ、FT-IR、NMR、UV-Vis分光法などの手法を使用して特徴付けることができます .
科学研究の応用
レパグリニドは、次のものなど、いくつかの科学研究の応用があります。
化学: 糖尿病治療薬の合成と改変を研究するためのモデル化合物として使用されます。
生物学: 膵臓のβ細胞とインスリン分泌への影響が研究されています。
医学: 2型糖尿病の治療における有効性と安全性を評価する臨床試験で使用されています。
類似化合物との比較
Repaglinide is often compared with other antidiabetic drugs, such as:
Nateglinide: Another meglitinide with a similar mechanism of action but a shorter duration of effect.
Sulfonylureas: Such as glibenclamide and glimepiride, which also stimulate insulin release but have a longer duration of action and higher risk of hypoglycemia.
Metformin: A biguanide that reduces hepatic glucose production and improves insulin sensitivity but does not stimulate insulin release
Repaglinide is unique in its rapid onset and short duration of action, making it particularly effective for controlling postprandial blood glucose levels without causing prolonged hypoglycemia .
特性
IUPAC Name |
2-ethoxy-4-[2-[[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H36N2O4/c1-4-33-25-17-20(12-13-22(25)27(31)32)18-26(30)28-23(16-19(2)3)21-10-6-7-11-24(21)29-14-8-5-9-15-29/h6-7,10-13,17,19,23H,4-5,8-9,14-16,18H2,1-3H3,(H,28,30)(H,31,32)/t23-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FAEKWTJYAYMJKF-QHCPKHFHSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC1=C(C=CC(=C1)CC(=O)NC(CC(C)C)C2=CC=CC=C2N3CCCCC3)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC1=C(C=CC(=C1)CC(=O)N[C@@H](CC(C)C)C2=CC=CC=C2N3CCCCC3)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H36N2O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3023552 | |
| Record name | Repaglinide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3023552 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
452.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Repaglinide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015048 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>67.9 [ug/mL] (The mean of the results at pH 7.4), 2.94e-03 g/L | |
| Record name | SID49648522 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Repaglinide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015048 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Repaglinide activity is dependent on the presence functioning β cells and glucose. In contrast to sulfonylurea insulin secretatogogues, repaglinide has no effect on insulin release in the absence of glucose. Rather, it potentiates the effect of extracellular glucose on ATP-sensitive potassium channel and has little effect on insulin levels between meals and overnight. As such, repaglinide is more effective at reducing postprandial blood glucose levels than fasting blood glucose levels and requires a longer duration of therapy (approximately one month) before decreases in fasting blood glucose are observed. The insulinotropic effects of repaglinide are highest at intermediate glucose levels (3 to 10 mmol/L) and it does not increase insulin release already stimulated by high glucose concentrations (greater than 15 mmol/L). Repaglinide appears to be selective for pancreatic β cells and does not appear to affect skeletal or cardiac muscle or thyroid tissue. | |
| Record name | Repaglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
135062-02-1 | |
| Record name | Repaglinide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=135062-02-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Repaglinide [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0135062021 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Repaglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Repaglinide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759893 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Repaglinide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3023552 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (+)-2-Ethoxy-alpha-(((S)-alpha-isobutyl-o-piperidinobenzyl)carbamoyl)-p-toluic acid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | REPAGLINIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/668Z8C33LU | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Repaglinide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015048 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
130-131 °C, 130 - 131 °C | |
| Record name | Repaglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00912 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Repaglinide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015048 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details






Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of repaglinide?
A: Repaglinide is a non-sulfonylurea insulin secretagogue. It works by binding to and blocking ATP-sensitive potassium (K-ATP) channels on the surface of pancreatic beta cells. [, , , , ] This blockage depolarizes the beta cells, leading to the opening of voltage-gated calcium channels. The influx of calcium ions triggers the release of insulin from the beta cells. [, , , ]
Q2: How does the action of repaglinide differ from sulfonylureas?
A: While both repaglinide and sulfonylureas stimulate insulin release by binding to the sulfonylurea receptor 1 (SUR1) subunit of the K-ATP channel, they do so at distinct binding sites. [] This difference translates to a faster onset and shorter duration of action for repaglinide compared to sulfonylureas. [, , , , , ]
Q3: What is the impact of repaglinide on insulin secretion patterns?
A: Repaglinide primarily amplifies the mass and amplitude of insulin secretory bursts without affecting the frequency of these bursts. [] This action enhances both early- and late-phase insulin secretion in response to hyperglycemia. []
Q4: What is the molecular formula and weight of repaglinide?
A: Repaglinide has the molecular formula C27H36N2O4 and a molecular weight of 452.58 g/mol. []
Q5: Is there any spectroscopic data available for repaglinide?
A: Yes, repaglinide shows maximum UV absorbance at a wavelength of 237 nm in both phosphate buffer (pH 7.4) and 0.1 N HCl solutions. [] Fourier-transform infrared spectroscopy (FT-IR) analysis reveals characteristic peaks for repaglinide. [, ]
Q6: How does co-administration of repaglinide with cytochrome P450 (CYP)3A4 inhibitors affect its pharmacokinetics?
A: Strong CYP3A4 inhibitors, like ketoconazole, can increase the area under the curve (AUC) and peak plasma concentration (Cmax) of repaglinide, although to a lesser extent than expected due to its metabolism by multiple CYP enzymes. [] This interaction may necessitate adjustments in repaglinide dosage and blood glucose monitoring. []
Q7: Does the co-administration of repaglinide with CYP3A4 inducers impact its effectiveness?
A: Rifampicin, a potent CYP3A4 inducer, can significantly decrease the AUC and Cmax of repaglinide. [, , ] This interaction can potentially reduce the glucose-lowering effects of repaglinide, requiring dose adjustments and close monitoring. []
Q8: How does trimethoprim affect the pharmacokinetics and pharmacodynamics of repaglinide?
A: Trimethoprim, a CYP2C8 inhibitor, can significantly increase the plasma concentrations of repaglinide by inhibiting its CYP2C8-mediated metabolism. [, ] This interaction may increase the risk of hypoglycemia, particularly at higher doses. [, ]
Q9: Does grapefruit juice consumption impact the pharmacokinetics of repaglinide?
A: Grapefruit juice, a known inhibitor of intestinal CYP3A4, can increase the bioavailability of repaglinide, potentially leading to higher plasma concentrations. [] This effect is more prominent at lower doses of repaglinide. []
Q10: What is the absorption profile of repaglinide?
A: Repaglinide is rapidly and completely absorbed from the gastrointestinal tract after oral administration. [, , ]
Q11: What is the time to peak plasma concentration (Tmax) for repaglinide?
A: Peak plasma levels (Cmax) are achieved within 1 hour (Tmax) of administration. [, ]
Q12: How is repaglinide metabolized and eliminated from the body?
A: Repaglinide is primarily metabolized in the liver by CYP enzymes, mainly CYP2C8 and CYP3A4, into inactive metabolites. [, , , ] These metabolites are subsequently excreted primarily in bile. [, ]
Q13: Does the presence of the CYP2C8*3 allele affect the pharmacokinetics of repaglinide?
A: Contrary to some previous studies, research indicates that the CYP2C8*3 allele does not significantly alter the pharmacokinetics of repaglinide at therapeutic doses. []
Q14: Does the SLCO1B1 gene, which encodes for the OATP1B1 transporter, influence repaglinide disposition?
A: While pitavastatin, an OATP1B1 inhibitor, can increase repaglinide Cmax in individuals with specific SLCO1B1 genotypes, it does not significantly affect its overall pharmacokinetics or pharmacodynamics. [, ]
Q15: What is the primary therapeutic use of repaglinide?
A: Repaglinide is primarily indicated for the treatment of type 2 diabetes mellitus, particularly in improving glycemic control. [, , , , , , ]
Q16: How does repaglinide compare to other antidiabetic agents in terms of efficacy?
A: In clinical trials, repaglinide demonstrated comparable efficacy to glibenclamide and gliclazide in improving glycemic control. [] It also showed superior efficacy to glipizide in maintaining glycemic control over a year. []
Q17: What is the role of repaglinide in combination therapy for type 2 diabetes?
A: Repaglinide is often used in combination with other antidiabetic agents, such as metformin or thiazolidinediones, to enhance glycemic control. [, , , , , , ]
Q18: Can repaglinide be used as monotherapy in the treatment of type 2 diabetes?
A: Yes, clinical trials have shown repaglinide monotherapy to be effective in improving glycemic control in patients with type 2 diabetes. []
Q19: What formulation strategies have been explored to improve the solubility and dissolution rate of repaglinide?
A: Solid dispersions of repaglinide with polymers like polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), mannitol, and urea have been investigated to enhance its solubility and dissolution rate. [, , , ]
Q20: Have any specific formulations, such as fast-dissolving tablets, been developed for repaglinide?
A: Yes, fast-dissolving tablets incorporating repaglinide solid dispersions and superdisintegrants have been developed to improve its bioavailability and potentially enhance its therapeutic efficacy. []
Q21: What is the rationale behind developing transdermal patches for repaglinide delivery?
A: Transdermal patches have been explored to achieve sustained release of repaglinide, improve patient compliance, and potentially reduce the frequency of administration. []
Q22: What are the most commonly reported adverse effects associated with repaglinide?
A: The most frequent adverse effects of repaglinide are hypoglycemia, upper respiratory tract infection, rhinitis, bronchitis, and headache. []
Q23: How does the risk of hypoglycemia with repaglinide compare to sulfonylureas?
A: Due to its shorter duration of action and meal-time dosing, repaglinide is associated with a lower risk of serious hypoglycemia compared to sulfonylureas, particularly if a meal is missed. [, , ]
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
![Methyl 6-methyl-3-(2-methylpropyl)-4-(3-nitrophenyl)-4,7-dihydrothieno[2,3-b]pyridine-5-carboxylate](/img/structure/B1680436.png)
![N-[4-methoxy-3-(4-methylpiperazin-1-yl)phenyl]-1,2-dihydrobenzo[e]indole-3-carboxamide;hydrochloride](/img/structure/B1680439.png)
![N-[4-[(3aR,9bS)-8-cyano-3,3a,4,9b-tetrahydro-1H-chromeno[3,4-c]pyrrol-2-yl]butyl]-4-phenylbenzamide](/img/structure/B1680441.png)