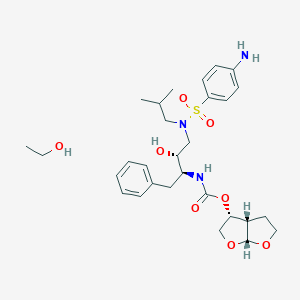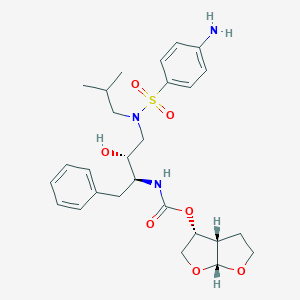
ダルナビル
概要
説明
ダルナビルは、主にヒト免疫不全ウイルス(HIV)感染の治療と予防に使用される、非ペプチド系プロテアーゼ阻害剤です。その有効性を高めるために、他の抗レトロウイルス剤と併用して投与されることがよくあります。 ダルナビルは、2006年に米国食品医薬品局(FDA)によって承認されており、世界保健機関(WHO)の必須医薬品リストに含まれています .
2. 製法
合成経路と反応条件: ダルナビルの合成には、主要な中間体の生成とその後のカップリングを含む、複数の工程が含まれます。 一般的な合成経路の1つは、ジクロロメタン中のトリフルオロ酢酸を用いてBOC基を除去し、続いてトリエチルアミン存在下で炭酸塩と反応させてダルナビルを生成する工程です .
工業的製造方法: ダルナビルエタノール塩の工業的製造には、複数の単離と乾燥工程を含む堅牢なプロセスが用いられます。 このプロセスは、高い化学的収率と純度を確保し、重要なプロセス不純物は所望の限界値以下に管理されています . さらに、ホットメルト押出成形や噴霧乾燥などの方法が、ダルナビルの溶解性とバイオアベイラビリティを向上させるために用いられています .
3. 化学反応解析
反応の種類: ダルナビルは、酸化、還元、置換などのさまざまな化学反応を起こします。 主にCYP3A4である肝臓のシトクロム酵素によって、強力に酸化され、代謝されます .
一般的な試薬と条件:
酸化: 肝臓のシトクロム酵素が含まれます。
還元: ダルナビルでは一般的に報告されていません。
置換: 炭酸塩とアミンとの反応が含まれます。
主な生成物: これらの反応から生成される主な生成物には、ヒドロキシル化およびグルクロン酸抱合代謝物があります .
4. 科学研究における用途
ダルナビルは、科学研究において幅広い用途があります:
化学: プロテアーゼ阻害剤の研究におけるモデル化合物として使用されます。
生物学: HIVプロテアーゼやその他の生物学的標的との相互作用について調査されています。
作用機序
ダルナビルは、ウイルス前駆体タンパク質の処理とウイルスの成熟に不可欠なHIV-1プロテアーゼ酵素を阻害することにより、その効果を発揮します。 ダルナビルは、酵素の活性部位に結合することにより、HIV Gag-Pol ポリタンパク質の切断を阻害し、感染性ビリオンの形成を阻害します . この機序は、その抗レトロウイルス活性にとって重要です。
類似化合物:
- アンプレナビル
- インジナビル
- サキナビル
- ネルフィナビル
- リトナビル
比較: ダルナビルは、高い耐性遺伝子バリアと、幅広いプロテアーゼ阻害剤耐性HIV-1株を阻害できることから、他のプロテアーゼ阻害剤とは異なります . 他のいくつかのプロテアーゼ阻害剤とは異なり、ダルナビルは、その薬物動態特性を向上させるために、リトナビルまたはコビシスタットと併用して使用されることが多いです .
科学的研究の応用
生化学分析
Biochemical Properties
Darunavir plays a crucial role in biochemical reactions by inhibiting the activity of the HIV-1 protease enzyme. This enzyme is responsible for cleaving the viral polyprotein into functional proteins necessary for viral replication . Darunavir binds to the active site of the HIV-1 protease through multiple hydrogen bonds, thereby preventing the enzyme from processing the viral polyprotein . This inhibition leads to the production of immature, non-infectious viral particles . Darunavir interacts with various biomolecules, including the HIV-1 protease enzyme and other proteins involved in the viral replication process .
Cellular Effects
Darunavir has significant effects on various types of cells and cellular processes. In HIV-infected cells, Darunavir reduces viral load and increases CD4 cell counts, which are crucial for maintaining a healthy immune system . By inhibiting the HIV-1 protease enzyme, Darunavir disrupts the viral replication cycle, leading to a decrease in the production of new viral particles . This inhibition also affects cell signaling pathways, gene expression, and cellular metabolism by preventing the virus from hijacking the host cell’s machinery .
Molecular Mechanism
The molecular mechanism of Darunavir involves its binding to the active site of the HIV-1 protease enzyme. Darunavir forms multiple hydrogen bonds with the enzyme, which stabilizes its binding and prevents the protease from cleaving the viral polyprotein . This inhibition results in the production of immature viral particles that are unable to infect new cells . Additionally, Darunavir’s high affinity for the protease enzyme makes it effective against HIV strains that have developed resistance to other protease inhibitors .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Darunavir have been observed to change over time. Darunavir is generally stable and maintains its efficacy over extended periods . Long-term studies have shown that resistance-associated mutations can emerge in patients experiencing virological failure during prolonged use of Darunavir . These mutations can reduce the drug’s effectiveness, necessitating adjustments in treatment regimens .
Dosage Effects in Animal Models
The effects of Darunavir vary with different dosages in animal models. Studies have shown that higher doses of Darunavir result in increased drug concentrations in the brain, liver, and plasma . At high doses, Darunavir can also cause toxic or adverse effects, such as gastrointestinal disturbances and lipid abnormalities . It is essential to determine the optimal dosage to maximize efficacy while minimizing adverse effects .
Metabolic Pathways
Darunavir is primarily metabolized by the cytochrome P450 3A (CYP3A) isoenzymes in the liver . The metabolic pathways involve carbamate hydrolysis, isobutyl aliphatic hydroxylation, and aniline aromatic hydroxylation . Ritonavir, a CYP3A inhibitor, is often co-administered with Darunavir to enhance its bioavailability and prolong its half-life . This combination allows for lower daily doses of Darunavir while maintaining its therapeutic efficacy .
Transport and Distribution
Darunavir is transported and distributed within cells and tissues through various mechanisms. It exhibits sufficient membrane permeability to achieve adequate intestinal absorption . The drug is also subject to active transport processes, such as those mediated by P-glycoprotein (P-gp) or other efflux proteins . These transporters play a role in the drug’s localization and accumulation within different tissues .
Subcellular Localization
The subcellular localization of Darunavir is primarily within the cytoplasm, where it interacts with the HIV-1 protease enzyme . Darunavir’s activity is not significantly affected by targeting signals or post-translational modifications, as its primary function is to inhibit the protease enzyme within the cytoplasmic compartment . This localization ensures that Darunavir effectively disrupts the viral replication process within infected cells .
準備方法
Synthetic Routes and Reaction Conditions: The synthesis of darunavir involves multiple steps, including the formation of key intermediates and their subsequent coupling. One common synthetic route includes the removal of the BOC group using trifluoroacetic acid in dichloromethane, followed by reaction with a carbonate in the presence of triethylamine to yield darunavir .
Industrial Production Methods: Industrial production of darunavir ethanolate involves a robust process with multiple isolations and drying steps. This process ensures high chemical yield and purity, with critical process impurities controlled below desired limits . Additionally, methods such as hot-melt extrusion and spray-drying are employed to improve the solubility and bioavailability of darunavir .
化学反応の分析
Types of Reactions: Darunavir undergoes various chemical reactions, including oxidation, reduction, and substitution. It is heavily oxidized and metabolized by hepatic cytochrome enzymes, primarily CYP3A4 .
Common Reagents and Conditions:
Oxidation: Involves hepatic cytochrome enzymes.
Reduction: Not commonly reported for darunavir.
Substitution: Involves reactions with carbonates and amines.
Major Products: The major products formed from these reactions include hydroxylated and glucuronidated metabolites .
類似化合物との比較
- Amprenavir
- Indinavir
- Saquinavir
- Nelfinavir
- Ritonavir
Comparison: Darunavir is unique among protease inhibitors due to its high genetic barrier to resistance and its ability to inhibit a wide range of protease inhibitor-resistant HIV-1 strains . Unlike some other protease inhibitors, darunavir is often used in combination with ritonavir or cobicistat to enhance its pharmacokinetic profile .
特性
IUPAC Name |
[(3aS,4R,6aR)-2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CJBJHOAVZSMMDJ-HEXNFIEUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)CN(CC(C(CC1=CC=CC=C1)NC(=O)OC2COC3C2CCO3)O)S(=O)(=O)C4=CC=C(C=C4)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)CN(C[C@H]([C@H](CC1=CC=CC=C1)NC(=O)O[C@H]2CO[C@@H]3[C@H]2CCO3)O)S(=O)(=O)C4=CC=C(C=C4)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H37N3O7S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0046779 | |
| Record name | Darunavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0046779 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
547.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Darunavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Approximately 0.15 mg/mL at, 6.68e-02 g/L | |
| Record name | Darunavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01264 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Darunavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The HIV-1 protease enzyme is necessary for viral precursor protein processing and viral maturation in preparation for infection, and is therefore a target for antiretroviral therapy for HIV. Protease inhibitors are used as a part of highly active antiretroviral therapy (HAART) in patients diagnosed with HIV infection. It has been shown to effectively suppress the virus, leading to significantly decreased morbidity and mortality rates. Darunavir, a HIV protease inhibitor, prevents HIV replication through binding to the enzyme, stopping the dimerization and the catalytic activity of HIV-1 protease. In particular, it inhibits the cleavage of HIV encoded Gag-Pol proteins in cells that have been infected with the virus, halting the formation of mature virus particles, which spread the infection. The close contact that darunavir makes with the primary chains of the active site amino acids (Asp-29 and Asp-30) on the protease likely contributes to its potency and efficacy against resistant variants of HIV-1. Darunavir is known to bind to different sites on the enzyme: the active site cavity and the surface of one of the flexible flaps in the protease dimer. Darunavir can adapt to changes in the shape of a protease enzyme due to its molecular flexibility., Darunavir as a protease inhibitor inhibits the cleavage of HIV encoded gag-pol polyproteins in virus infected cells, thereby preventing the formation of mature and infectious new virions. It was selected for its potency against wild type HIV-1 and HIV strains resistant to currently approved protease inhibitors., Darunavir is an inhibitor of the HIV-1 protease. It selectively inhibits the cleavage of HIV encoded Gag-Pol polyproteins in infected cells, thereby preventing the formation of mature virus particles. | |
| Record name | Darunavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01264 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Darunavir | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7788 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White, amorphous solid | |
CAS No. |
206361-99-1 | |
| Record name | Darunavir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=206361-99-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Darunavir [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0206361991 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Darunavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01264 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Darunavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0046779 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Carbamic acid, N-[(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-, (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl ester | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DARUNAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/YO603Y8113 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Darunavir | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7788 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Darunavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
74-76, 74 °C (decomposes) | |
| Record name | Darunavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01264 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Darunavir | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7788 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。