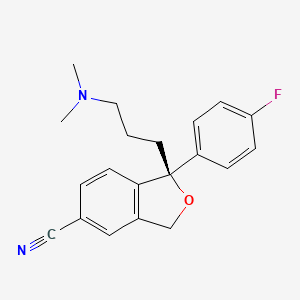
Escitalopram
Übersicht
Beschreibung
Escitalopram ist ein selektiver Serotonin-Wiederaufnahmehemmer, der hauptsächlich als Antidepressivum eingesetzt wird. Es ist das S-Enantiomer von Citalopram, d. h. es ist eine der beiden spiegelbildlichen Formen des Moleküls. This compound ist bekannt für seine hohe Selektivität und Potenz bei der Hemmung des Serotonintransporters, wodurch es wirksam bei der Behandlung von Major Depressive Disorder und generalisierter Angststörung ist .
Herstellungsmethoden
Synthesewege und Reaktionsbedingungen
Die Synthese von this compound umfasst mehrere Schritte, ausgehend vom Vorläufer Citalopram. Eine übliche Methode umfasst die Trennung des racemischen Gemisches von Citalopram in seine Enantiomere unter Verwendung der chiralen Chromatographie. Das S-Enantiomer wird dann isoliert und gereinigt .
Eine andere Methode beinhaltet die Cyclisierung einer Diolverbindung, gefolgt von der Trennung der resultierenden Verbindung. Die Diolverbindung wird oft als Oxalatsalz hergestellt, um den Trennungsprozess zu erleichtern .
Industrielle Produktionsmethoden
Die industrielle Produktion von this compound folgt typischerweise denselben Synthesewegen, jedoch in größerem Maßstab. Die Verwendung chiraler stationärer Phasen wie Chiralpak AD oder Chiralcel OD ist üblich bei der chromatographischen Trennung von Enantiomeren . Der Prozess ist optimiert, um eine hohe Ausbeute und Reinheit des Endprodukts zu gewährleisten.
Wirkmechanismus
Target of Action
Escitalopram, a selective serotonin reuptake inhibitor (SSRI), primarily targets the serotonin transporter (SERT) . SERT is responsible for the reuptake of serotonin (5-hydroxytryptamine [5-HT]) at the terminals and cell bodies of serotonergic neurons .
Mode of Action
This compound binds with high affinity to the 5-HT binding site (orthosteric site) on the SERT . This enhancement of serotonergic activity is believed to be the mechanism underlying its antidepressant and anxiolytic effects .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the serotonergic neurotransmission pathway . By inhibiting the reuptake of serotonin, this compound increases extracellular 5-HT levels, which enhances 5-HT neurotransmission .
Pharmacokinetics
This compound exhibits good bioavailability (approximately 80%) after oral administration . It is metabolized in the liver, specifically by the enzymes CYP3A4 and CYP2C19, into S-desmethylcitalopram . The elimination half-life of this compound is approximately 27–32 hours . These properties influence the drug’s bioavailability and therapeutic effects.
Result of Action
The increased serotonergic activity resulting from this compound’s action leads to improved mood and reduced symptoms of depression and anxiety . This is why this compound is used in the treatment of major depressive disorder (MDD), generalized anxiety disorder (GAD), and other select psychiatric disorders such as obsessive-compulsive disorder (OCD) .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, the presence of certain concomitant medications can affect the metabolism of this compound, potentially altering its therapeutic effects . Additionally, genetic factors such as variations in the CYP2C19 gene can affect the metabolism of this compound, leading to differences in drug exposure among individuals .
Wissenschaftliche Forschungsanwendungen
Escitalopram has a wide range of scientific research applications:
Chemistry: It is used as a model compound in the study of chiral separation techniques and enantiomeric purity.
Biology: Research on this compound includes its effects on neurotransmitter systems and its role in neuroplasticity.
Medicine: this compound is extensively studied for its efficacy in treating depression, anxiety, and other psychiatric disorders. .
Biochemische Analyse
Biochemical Properties
The primary molecular target for Escitalopram is the serotonin transporter (SERT), which is responsible for serotonin (also called 5-hydroxytryptamine [5-HT]) reuptake at the terminals and cell bodies of serotonergic neurons . This leads to antidepressant effects by increasing extracellular 5-HT levels which enhance 5-HT neurotransmission .
Cellular Effects
This compound exerts its effects by restoring serotonergic function in the treatment of depression and anxiety . It is approximately 150 times more potent than citalopram’s R-enantiomer and is responsible for the vast majority of citalopram’s clinical activity .
Molecular Mechanism
This compound exerts its effects at the molecular level through binding interactions with the serotonin transporter (SERT). It has been shown that through allosteric binding, this compound decreases its own dissociation rate from the orthosteric site on the SERT . This may be the mechanism responsible for its observed superior efficacy and faster onset compared to other SSRIs .
Metabolic Pathways
This compound is involved in the serotonin reuptake metabolic pathway. It interacts with the serotonin transporter (SERT), affecting the reuptake of serotonin at the terminals and cell bodies of serotonergic neurons .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions
The synthesis of escitalopram involves several steps, starting from the precursor citalopram. One common method includes the resolution of the racemic mixture of citalopram into its enantiomers using chiral chromatography. The S-enantiomer is then isolated and purified .
Another method involves the cyclization of a diol compound, followed by the resolution of the resulting compound. The diol compound is often prepared as an oxalate salt to facilitate the resolution process .
Industrial Production Methods
Industrial production of this compound typically follows the same synthetic routes but on a larger scale. The use of chiral stationary phases such as Chiralpak AD or Chiralcel OD is common in the chromatographic separation of enantiomers . The process is optimized to ensure high yield and purity of the final product.
Analyse Chemischer Reaktionen
Arten von Reaktionen
Escitalopram unterliegt verschiedenen Arten von chemischen Reaktionen, darunter:
Oxidation: this compound kann oxidiert werden, um verschiedene Metaboliten zu bilden.
Reduktion: Reduktionsreaktionen sind weniger häufig, können aber unter bestimmten Bedingungen auftreten.
Substitution: Substitutionsreaktionen, insbesondere unter Beteiligung der Cyanogruppe, sind für die Synthese von this compound-Analoga von Bedeutung.
Häufige Reagenzien und Bedingungen
Oxidation: Häufige Oxidationsmittel umfassen Kaliumpermanganat und Wasserstoffperoxid.
Reduktion: Reduktionsmittel wie Lithiumaluminiumhydrid können verwendet werden.
Substitution: Reagenzien wie Natriumcyanid und Palladiumkatalysatoren werden bei Substitutionsreaktionen verwendet.
Hauptprodukte
Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, umfassen verschiedene Metaboliten und Analoga von this compound, die oft auf ihre pharmakologischen Eigenschaften untersucht werden .
Wissenschaftliche Forschungsanwendungen
This compound hat eine große Bandbreite an wissenschaftlichen Forschungsanwendungen:
Chemie: Es wird als Modellverbindung in der Untersuchung von chiralen Trenntechniken und enantiomerer Reinheit verwendet.
Biologie: Die Forschung an this compound umfasst seine Auswirkungen auf Neurotransmittersysteme und seine Rolle bei der Neuroplastizität.
Medizin: this compound wird ausgiebig auf seine Wirksamkeit bei der Behandlung von Depression, Angstzuständen und anderen psychiatrischen Erkrankungen untersucht. .
Wirkmechanismus
This compound wirkt, indem es die Wiederaufnahme von Serotonin, einem Neurotransmitter, in das präsynaptische Neuron hemmt. Diese Hemmung erhöht den Serotonin-Spiegel, der im synaptischen Spalt verfügbar ist, wodurch die serotonerge Neurotransmission verstärkt wird. Das primäre molekulare Ziel ist der Serotonintransporter (SERT), an den this compound an der orthosteren Stelle bindet und die Serotonin-Wiederaufnahme verhindert .
Vergleich Mit ähnlichen Verbindungen
Ähnliche Verbindungen
Citalopram: Das racemische Gemisch, aus dem Escitalopram gewonnen wird.
Fluoxetin: Ein weiterer selektiver Serotonin-Wiederaufnahmehemmer mit einer anderen chemischen Struktur.
Sertralin: Ein selektiver Serotonin-Wiederaufnahmehemmer mit einem ähnlichen Wirkmechanismus, aber unterschiedlichen pharmakokinetischen Eigenschaften
Einzigartigkeit
This compound ist einzigartig aufgrund seiner hohen Selektivität und Potenz für den Serotonintransporter. Es hat sich in einigen klinischen Studien als wirksamer und besser verträglich erwiesen als sein racemisches Gemisch, Citalopram, und andere selektive Serotonin-Wiederaufnahmehemmer . Seine allosterische Modulation des Serotonintransporters trägt ebenfalls zu seiner überlegenen Wirksamkeit und schnelleren Wirkungseintritt im Vergleich zu anderen selektiven Serotonin-Wiederaufnahmehemmern bei .
Eigenschaften
IUPAC Name |
(1S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3H-2-benzofuran-5-carbonitrile | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3/t20-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WSEQXVZVJXJVFP-FQEVSTJZSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCCC1(C2=C(CO1)C=C(C=C2)C#N)C3=CC=C(C=C3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN(C)CCC[C@@]1(C2=C(CO1)C=C(C=C2)C#N)C3=CC=C(C=C3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H21FN2O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8048440 | |
| Record name | Escitalopram | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8048440 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
324.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Escitalopram | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005028 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Sparingly soluble | |
| Record name | Escitalopram | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01175 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Escitalopram, like other selective serotonin re-uptake inhibitors, enhances serotonergic activity by binding to the orthosteric (i.e. primary) binding site on the serotonin transporter (SERT), the same site to which endogenous 5-HT binds, and thus prevents the re-uptake of serotonin into the presynaptic neuron. Escitalopram, along with [paroxetine], is also considered an allosteric serotonin re-uptake inhibitor - it binds to a secondary allosteric site on the SERT molecule to more strongly inhibit 5-HT re-uptake. Its combination of orthosteric and allosteric activity on SERT allows for greater extracellular 5-HT levels, a faster onset of action, and greater efficacy as compared to other SSRIs. The sustained elevation of synaptic 5-HT eventually causes desensitization of 5-HT1A auto-receptors, which normally shut down endogenous 5-HT release in the presence of excess 5-HT - this desensitization may be necessary for the full clinical effect of SSRIs and may be responsible for their typically prolonged onset of action. Escitalopram has shown little-to-no binding affinity at a number of other receptors, such as histamine and muscarinic receptors, and minor activity at these off-targets may explain some of its adverse effects., The mechanism of antidepressant action of escitalopram, the S-enantiomer of racemic citalopram, is presumed to be linked to potentiation of serotonergic activity in the central nervous system (CNS) resulting from its inhibition of CNS neuronal reuptake of serotonin (5-HT). | |
| Record name | Escitalopram | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01175 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Escitalopram | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8410 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
128196-01-0 | |
| Record name | (+)-Citalopram | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=128196-01-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Escitalopram [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0128196010 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Escitalopram | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01175 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Escitalopram | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8048440 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-2-benzofuran-5-carbonitrile | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.244.188 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ESCITALOPRAM | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4O4S742ANY | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Escitalopram | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8410 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Escitalopram | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005028 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
147-152C | |
| Record name | Escitalopram | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01175 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details







Synthesis routes and methods IV
Procedure details










Synthesis routes and methods V
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of escitalopram?
A: this compound is a selective serotonin reuptake inhibitor (SSRI). It exerts its therapeutic effect by blocking the serotonin transporter (SERT) in the central nervous system (CNS). [, , , ] This inhibition increases the extracellular concentration of serotonin in the synaptic cleft, enhancing serotonergic neurotransmission.
Q2: What is the molecular formula and weight of this compound?
A: this compound's molecular formula is C20H21FN2O, and its molecular weight is 324.4 g/mol. []
Q3: Is there any spectroscopic data available for this compound?
A: While specific spectroscopic data wasn't provided in these papers, analytical techniques like HPLC-MS/MS are commonly employed for its characterization and quantification. [, ]
Q4: Is there information available regarding the material compatibility and stability of this compound under various conditions?
A4: The provided research papers primarily focus on the pharmacological aspects of this compound rather than its material properties. Therefore, information on material compatibility and stability is limited within these sources.
Q5: Does this compound possess any catalytic properties or find applications in catalysis?
A5: this compound is primarily recognized for its pharmacological activity as an antidepressant. The provided papers do not suggest any catalytic properties or applications for this compound.
Q6: Have computational chemistry methods been employed to study this compound?
A: While not extensively discussed in these papers, computational chemistry techniques, including QSAR modeling, can be valuable for exploring the structure-activity relationships of this compound and designing potential analogs. []
Q7: What is the significance of this compound being the S-enantiomer of citalopram?
A: this compound is the active S-enantiomer of the racemic drug citalopram. Research has shown that the R-enantiomer of citalopram may counteract the therapeutic effects of the S-enantiomer. [] Therefore, this compound, as a single enantiomer, demonstrates enhanced efficacy and potentially fewer side effects compared to the racemate. [, , ]
Q8: What is the primary route of metabolism for this compound?
A: this compound is primarily metabolized in the liver by the cytochrome P450 (CYP) enzyme system, specifically CYP2C19. [, ] Polymorphisms in the CYP2C19 gene can significantly influence this compound plasma concentrations and potentially affect individual treatment response. [, , ]
Q9: How does the co-administration of fluvoxamine, a CYP2C19 inhibitor, impact this compound levels?
A: Co-administration of fluvoxamine significantly increases plasma concentrations of this compound by inhibiting its metabolism through CYP2C19. [] This interaction highlights the importance of considering potential drug interactions when prescribing this compound.
Q10: Are there any known drug transporter interactions associated with this compound?
A: While specific drug transporter interactions are not extensively covered in these papers, it's important to be aware that drug transporters play a crucial role in drug absorption, distribution, and elimination. [] Further research may provide more insights into potential transporter interactions with this compound.
Q11: Has the efficacy of this compound been demonstrated in preclinical models of depression?
A: Yes, studies using the chronic mild stress (CMS) model in rats have shown that this compound effectively alleviates depressive-like behaviors. [] This model is commonly used to assess the efficacy of potential antidepressant compounds.
Q12: What do clinical trials indicate about the efficacy of this compound in treating major depressive disorder (MDD)?
A: Numerous clinical trials have consistently demonstrated the efficacy of this compound in treating MDD. [, , , ] Meta-analyses comparing this compound to other SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have confirmed its superior efficacy in improving depressive symptoms and achieving remission. [, ]
Q13: Does this compound demonstrate efficacy in treating conditions other than MDD?
A: Besides MDD, clinical trials have supported the efficacy of this compound in treating generalized anxiety disorder (GAD). [] Additionally, some research suggests its potential benefit in managing other conditions, such as obsessive-compulsive disorder (OCD). []
Q14: Is there evidence of resistance developing to this compound treatment?
A: While the development of resistance to this compound is not extensively discussed in these papers, it's a phenomenon observed with many antidepressant medications. Further research is needed to understand the mechanisms underlying potential resistance to this compound. []
Q15: What are the common adverse effects associated with this compound?
A15: As this Q&A focuses on the scientific aspects and not clinical guidelines, we'll refrain from listing specific side effects. Please refer to the drug information leaflet or consult a healthcare professional for comprehensive information on this compound's adverse effects.
Q16: Are there any long-term safety concerns associated with this compound use?
A16: We recommend referring to the drug information leaflet or consulting a healthcare professional for information on the long-term safety of this compound.
Q17: Are there any specific drug delivery systems or targeting strategies being investigated for this compound?
A17: The provided research primarily focuses on the pharmacological effects of this compound, and these papers do not delve into specific drug delivery systems or targeting strategies being explored for this compound.
Q18: Are there any known biomarkers associated with this compound efficacy or adverse effects?
A: While specific biomarkers for this compound response are not extensively discussed in these papers, research on identifying potential biomarkers for antidepressant response is ongoing. [] Such biomarkers could potentially guide treatment selection and personalize therapy.
Q19: What analytical techniques are commonly used to quantify this compound in biological samples?
A: High-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) is a widely used method for the sensitive and specific quantification of this compound in plasma samples. [, , ] This technique enables accurate measurement of drug concentrations for pharmacokinetic studies and therapeutic drug monitoring.
Q20: Is there information available regarding the environmental impact and degradation of this compound?
A20: The provided research focuses on the pharmacological and clinical aspects of this compound. Therefore, information on its environmental impact and degradation pathways is limited within these sources.
Q21: Are there established protocols for validating analytical methods used to measure this compound concentrations?
A: Yes, rigorous analytical method validation is crucial for ensuring the accuracy, precision, and reliability of drug concentration measurements. [] Regulatory agencies, such as the FDA and EMA, provide guidelines for validating analytical methods used in pharmaceutical analysis.
Q22: What quality control measures are implemented during the manufacturing and distribution of this compound to ensure its quality, safety, and efficacy?
A: Stringent quality control and assurance measures are implemented throughout the entire lifecycle of this compound, from development to manufacturing and distribution. [] These measures ensure that the drug product consistently meets predefined quality standards and regulatory requirements.
Q23: Is there evidence indicating that this compound induces significant immunogenicity or immunological responses?
A23: The provided research papers do not focus on the immunogenic potential of this compound. Therefore, specific information on its immunogenicity and potential immunological responses is limited within these sources.
Q24: Does this compound induce or inhibit drug-metabolizing enzymes, potentially leading to drug interactions?
A: While this compound is primarily metabolized by CYP2C19, its potential to induce or inhibit other drug-metabolizing enzymes is not extensively discussed in these papers. [, ] It's generally advisable to consult drug interaction databases and resources for a comprehensive assessment of potential interactions with this compound.
Q25: Is there information on the biocompatibility and biodegradability of this compound?
A: The research papers provided primarily focus on the pharmacological and clinical aspects of this compound. As a result, specific details regarding its biocompatibility and biodegradability are not extensively covered in these sources. []
Q26: What are some alternative antidepressants available, and how does this compound compare in terms of efficacy, safety, and cost?
A: Several other SSRIs, SNRIs, and other classes of antidepressants are available as alternative treatment options. [, , ] The choice of antidepressant is typically individualized based on factors such as patient characteristics, medical history, potential drug interactions, and cost.
Q27: Are there specific guidelines or recommendations for the recycling and waste management of this compound?
A: Information regarding the recycling and waste management of this compound is not directly addressed in the provided research papers. Proper disposal of unused medications, often through take-back programs or following local regulations, is essential to minimize environmental risks. []
Q28: What research infrastructure and resources are essential for advancing our understanding of this compound and developing new antidepressants?
A: A robust research infrastructure, including well-equipped laboratories, access to clinical trial networks, and collaborations between academia and industry, is crucial for advancing antidepressant research. [] Additionally, funding agencies play a vital role in supporting research efforts.
Q29: What are some key historical milestones in the development and understanding of this compound and SSRIs in general?
A: The discovery and development of SSRIs, including this compound, represent significant milestones in the treatment of depression and anxiety disorders. [] These medications offered a more favorable safety and tolerability profile compared to older classes of antidepressants.
Q30: How has cross-disciplinary collaboration contributed to research on this compound and the development of new antidepressants?
A: Cross-disciplinary collaboration, involving pharmacologists, chemists, psychiatrists, neuroscientists, and other experts, has been essential for advancing antidepressant research. [] Such collaborations facilitate a more comprehensive understanding of depression, its underlying mechanisms, and the development of innovative treatment strategies.
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.