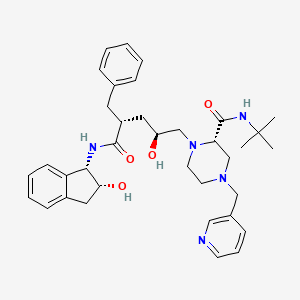
Indinavir
Übersicht
Beschreibung
Indinavir, bekannt unter dem Handelsnamen Crixivan, ist ein Proteaseinhibitor, der als Bestandteil einer hoch aktiven antiretroviralen Therapie zur Behandlung von HIV/AIDS eingesetzt wird . Es handelt sich um ein weißes, lösliches Pulver, das oral in Kombination mit anderen antiviralen Medikamenten verabreicht wird. This compound wurde synthetisch hergestellt, um das Proteaseenzym im HIV-Virus zu hemmen, wodurch die Vermehrung des Virus verhindert und die Viruslast bei Patienten reduziert wird .
2. Herstellungsmethoden
Synthesewege und Reaktionsbedingungen: this compound wird durch einen mehrstufigen Prozess synthetisiert, der die Bildung mehrerer Zwischenprodukte beinhaltetDie Reaktionsbedingungen beinhalten typischerweise die Verwendung von organischen Lösungsmitteln, Katalysatoren und kontrollierten Temperaturen, um sicherzustellen, dass die gewünschten chemischen Umwandlungen effizient ablaufen .
Industrielle Produktionsmethoden: Die industrielle Produktion von this compound beinhaltet die großtechnische Synthese unter optimierten Reaktionsbedingungen, um die Ausbeute und Reinheit zu maximieren. Der Prozess umfasst strenge Reinigungsschritte wie Kristallisation und Chromatographie, um das Endprodukt in reiner Form zu erhalten. Die Produktion erfolgt unter strengen Qualitätskontrollen, um Konsistenz und Sicherheit zu gewährleisten .
Wissenschaftliche Forschungsanwendungen
Indinavir hat ein breites Spektrum an Anwendungen in der wissenschaftlichen Forschung, darunter:
Chemie: this compound wird als Modellverbindung in Studien über Proteaseinhibitoren und deren Wechselwirkungen mit Enzymen verwendet.
Biologie: Es wird verwendet, um die Mechanismen der Virusreplikation und die Rolle von Proteasen in viralen Lebenszyklen zu untersuchen.
Medizin: this compound ist ein wichtiger Bestandteil der antiretroviralen Therapie bei HIV/AIDS und trägt dazu bei, die Krankheit zu kontrollieren und die Patientenergebnisse zu verbessern.
5. Wirkmechanismus
This compound hemmt das HIV-virale Proteaseenzym, das für die proteolytische Spaltung viraler Polyproteinvorläufer in einzelne funktionelle Proteine essentiell ist. Durch die Bindung an die aktive Stelle der Protease verhindert this compound die Spaltung dieser Polyproteine, was zur Bildung unreifer, nicht infektiöser Viruspartikel führt. Diese Hemmung reduziert die Viruslast bei Patienten und verlangsamt das Fortschreiten von HIV/AIDS .
Ähnliche Verbindungen:
- Ritonavir
- Saquinavir
- Nelfinavir
- Lopinavir
Vergleich: this compound ist unter den Proteaseinhibitoren einzigartig aufgrund seiner spezifischen Bindungsaffinität und seines Hemmmechanismus. Während andere Proteaseinhibitoren wie Ritonavir und Saquinavir ebenfalls das HIV-Proteaseenzym angreifen, ermöglicht die Molekülstruktur von this compound unterschiedliche Wechselwirkungen mit der aktiven Stelle des Enzyms, was zu unterschiedlichen pharmakokinetischen und pharmakodynamischen Profilen führt. Darüber hinaus werden die Löslichkeit und Bioverfügbarkeit von this compound durch seine spezifischen chemischen Modifikationen verbessert .
Die einzigartigen Eigenschaften von this compound machen es trotz seiner Nebenwirkungen und der Entwicklung von Resistenzen in einigen Fällen zu einem wertvollen Werkzeug bei der Behandlung von HIV/AIDS. Der Vergleich mit anderen Proteaseinhibitoren unterstreicht die Bedeutung der strukturellen Vielfalt bei der Entwicklung wirksamer antiretroviraler Therapien .
Wirkmechanismus
Target of Action
Indinavir, a potent and specific HIV protease inhibitor , primarily targets the HIV-1 protease , an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1 .
Mode of Action
This compound binds to the protease active site and inhibits the activity of the enzyme . This inhibition prevents the cleavage of the gag-pol polyprotein, resulting in the formation of immature, non-infectious viral particles .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the HIV life cycle . By inhibiting the HIV-1 protease, this compound disrupts the virus’s ability to process the polyproteins that are necessary for its replication . This results in the production of immature, non-infectious viral particles, thereby reducing the viral load .
Pharmacokinetics
This compound is administered orally and has a bioavailability of approximately 65% . It is metabolized in the liver via the CYP3A4 enzyme . The elimination half-life of this compound is approximately 1.8 ± 0.4 hours . These pharmacokinetic properties impact the drug’s bioavailability and necessitate frequent dosing .
Result of Action
The primary result of this compound’s action is a decrease in the viral load. By preventing the HIV-1 protease from functioning normally, this compound inhibits the replication of the HIV virus, leading to a decrease in the number of infectious particles .
Action Environment
The action of this compound can be influenced by various environmental factors. For instance, the drug’s absorption can be affected by food intake, and its metabolism can be influenced by the presence of other drugs that are metabolized by the same liver enzyme, CYP3A4 . Furthermore, the drug’s stability and efficacy can be affected by storage conditions .
Biochemische Analyse
Biochemical Properties
Indinavir interacts with HIV-1 protease, an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1 . The interaction between this compound and HIV-1 protease inhibits the activity of the enzyme .
Cellular Effects
This compound influences cell function by preventing the cleavage of the viral polyproteins, resulting in the formation of immature non-infectious viral particles . This impacts cell signaling pathways, gene expression, and cellular metabolism.
Molecular Mechanism
This compound exerts its effects at the molecular level by binding to the protease active site, inhibiting the enzyme’s activity . This inhibition prevents the cleavage of the viral polyproteins, leading to the formation of immature non-infectious viral particles .
Temporal Effects in Laboratory Settings
It is known that this compound is a potent and specific HIV protease inhibitor .
Metabolic Pathways
This compound is metabolized in the body, with seven metabolites identified, one glucuronide conjugate and six oxidative metabolites . Cytochrome P-450 3A4 (CYP3A4) is the major enzyme responsible for the formation of these oxidative metabolites .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Indinavir is synthesized through a multi-step process involving the formation of several intermediatesThe reaction conditions typically involve the use of organic solvents, catalysts, and controlled temperatures to ensure the desired chemical transformations occur efficiently .
Industrial Production Methods: Industrial production of this compound involves large-scale synthesis using optimized reaction conditions to maximize yield and purity. The process includes rigorous purification steps such as crystallization and chromatography to obtain the final product in its pure form. The production is carried out under strict quality control measures to ensure consistency and safety .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Indinavir unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann unter bestimmten Bedingungen oxidiert werden, was zur Bildung oxidierter Derivate führt.
Reduktion: Reduktionsreaktionen können die funktionellen Gruppen innerhalb von this compound modifizieren und möglicherweise dessen Aktivität verändern.
Häufige Reagenzien und Bedingungen:
Oxidation: Häufige Oxidationsmittel sind Wasserstoffperoxid und Kaliumpermanganat.
Reduktion: Reduktionsmittel wie Natriumborhydrid und Lithiumaluminiumhydrid werden verwendet.
Substitution: Verschiedene Nucleophile und Elektrophile können in Substitutionsreaktionen verwendet werden, abhängig von der gewünschten Modifikation.
Hauptprodukte, die gebildet werden: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, hängen von den spezifischen Reagenzien und Bedingungen ab, die verwendet werden. Beispielsweise kann Oxidation zur Bildung von hydroxylierten oder Keton-Derivaten führen, während Reduktion Alkohole oder Amine erzeugen kann .
Vergleich Mit ähnlichen Verbindungen
- Ritonavir
- Saquinavir
- Nelfinavir
- Lopinavir
Comparison: Indinavir is unique among protease inhibitors due to its specific binding affinity and inhibition mechanism. While other protease inhibitors like ritonavir and saquinavir also target the HIV protease enzyme, this compound’s molecular structure allows for distinct interactions with the enzyme’s active site, leading to different pharmacokinetic and pharmacodynamic profiles. Additionally, this compound’s solubility and bioavailability are enhanced by its specific chemical modifications .
This compound’s unique properties make it a valuable tool in the treatment of HIV/AIDS, despite its side effects and the development of resistance in some cases. Its comparison with other protease inhibitors highlights the importance of structural diversity in developing effective antiretroviral therapies .
Eigenschaften
IUPAC Name |
(2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-5-[[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino]-5-oxopentyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CBVCZFGXHXORBI-PXQQMZJSSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)(C)NC(=O)C1CN(CCN1CC(CC(CC2=CC=CC=C2)C(=O)NC3C(CC4=CC=CC=C34)O)O)CC5=CN=CC=C5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)(C)NC(=O)[C@@H]1CN(CCN1C[C@H](C[C@@H](CC2=CC=CC=C2)C(=O)N[C@@H]3[C@@H](CC4=CC=CC=C34)O)O)CC5=CN=CC=C5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C36H47N5O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
157810-81-6 (sulfate (1:1) (salt)) | |
| Record name | Indinavir [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0150378179 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID4043802 | |
| Record name | Indinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4043802 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
613.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Indinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014369 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
4.82e-02 g/L | |
| Record name | Indinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00224 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Indinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014369 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Indinavir inhibits the HIV viral protease enzyme which prevents cleavage of the gag-pol polyprotein, resulting in noninfectious, immature viral particles. | |
| Record name | Indinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00224 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
150378-17-9 | |
| Record name | Indinavir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=150378-17-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Indinavir [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0150378179 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Indinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00224 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Indinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4043802 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | INDINAVIR ANHYDROUS | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/9MG78X43ZT | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Indinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014369 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
167.5-168 °C, 167.5 - 168 °C | |
| Record name | Indinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00224 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Indinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014369 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![2-[(3,5-Dimethyl-1,2-oxazol-4-yl)methylsulfanyl]-1-[4-(2,3-dimethylphenyl)piperazin-1-yl]ethanone](/img/structure/B1671809.png)
![9,10-Dihydro-9,10[1',2']-benzenoanthracene-1,4-dione](/img/structure/B1671815.png)
