Anastrozol
Übersicht
Beschreibung
- Insbesondere ist es bei der Behandlung von hormonrezeptor-positivem Brustkrebs wirksam. Darüber hinaus wurde es als präventive Maßnahme für Personen mit hohem Brustkrebsrisiko eingesetzt .
Anastrozol: ist ein antiöstrogenes Medikament, das zur Klasse der Aromatasehemmer gehört. Es wird üblicherweise unter dem Markennamen verkauft und wird zusammen mit anderen Behandlungen bei Brustkrebs eingesetzt.
Herstellungsmethoden
Synthesewege: this compound wird in einem mehrstufigen Prozess synthetisiert. Der Schlüsselschritt beinhaltet die Kupplung von 1,2,4-Triazol mit einer Phenylmethylgruppe, gefolgt von Nitriladition. Die gesamte Reaktionssequenz führt zur Bildung der Verbindung.
Industrielle Produktion: this compound wird industriell unter Verwendung effizienter Synthesewege hergestellt.
Wirkmechanismus
Target of Action
Anastrozole is a competitive, selective, non-steroidal aromatase inhibitor . Its primary target is the enzyme aromatase, which plays a crucial role in the biosynthesis of estrogen . Aromatase catalyzes the conversion of androgens to estrogen . By inhibiting this enzyme, Anastrozole effectively decreases circulating estrogen levels .
Mode of Action
Anastrozole works by selectively inhibiting aromatase . It binds to the active site of the enzyme and prevents it from interacting with its substrate, thereby blocking the conversion of androgens to estrogens . This leads to a decrease in estrogen levels, particularly in postmenopausal women, where the primary source of estrogen is through the aromatization of androgens .
Biochemical Pathways
The key biochemical pathway affected by Anastrozole is the conversion of androgens to estrogens, catalyzed by aromatase . By inhibiting this enzyme, Anastrozole disrupts this pathway, leading to a decrease in estrogen levels . This is particularly significant in estrogen-responsive breast cancer, where estrogen plays a role in tumor growth and progression .
Pharmacokinetics
Anastrozole is primarily metabolized in the liver via oxidation and glucuronidation to a number of inactive metabolites . Its clearance is mainly via hepatic metabolism and can therefore be altered in patients with hepatic impairment . Patients with stable hepatic cirrhosis exhibit an apparent oral clearance approximately 30% lower compared with patients with normal liver function . Conversely, renal impairment has a negligible effect on total drug clearance as the renal route is a relatively minor clearance pathway for Anastrozole .
Result of Action
The molecular effect of Anastrozole’s action is the reduction of estrogen levels in the body . On a cellular level, this leads to a decrease in the growth and proliferation of hormone-dependent breast cancer cells . By reducing the levels of estrogen, Anastrozole deprives these cells of a key growth stimulus, thereby inhibiting tumor growth .
Action Environment
The action of Anastrozole can be influenced by environmental factors. For instance, patients with hepatic impairment may have altered drug clearance, affecting the drug’s efficacy . Furthermore, according to an environmental risk assessment, the use of Anastrozole is predicted to present an insignificant risk to the environment . The Predicted Environmental Concentration (PEC) / Predicted No Effect Concentration (PNEC) ratio is 1.4x10-4, indicating that the environmental impact of Anastrozole is minimal .
Wissenschaftliche Forschungsanwendungen
Chemie: Anastrozol ist ein wertvolles Werkzeug in der medizinischen Chemie, insbesondere im Bereich der Krebsforschung.
Biologie: Forscher untersuchen seine Auswirkungen auf den Östrogenstoffwechsel und seine Auswirkungen auf hormonabhängige Krebsarten.
Medizin: Über die Behandlung von Brustkrebs hinaus wird this compound für andere hormonbedingte Erkrankungen untersucht.
Wirkmechanismus
Östrogenunterdrückung: this compound hemmt das Enzym Aromatase, das Androgene in Östrogene umwandelt. Durch die Blockierung dieses Prozesses reduziert es den Östrogenspiegel im Körper.
Molekulare Ziele: Aromatase selbst ist das primäre molekulare Ziel. Durch seine Hemmung stört this compound die Östrogensynthese.
Beteiligte Wege: Die Verbindung wirkt sich auf östrogenabhängige Wege aus und beeinflusst das Zellwachstum und die Zellproliferation in hormonrezeptor-positiven Brustkrebszellen.
Biochemische Analyse
Biochemical Properties
Anastrozole works by inhibiting the enzyme aromatase, which is responsible for the conversion of androgens to estrogens in peripheral tissues . This inhibition prevents the synthesis of estrogen from adrenal androgens, primarily androstenedione and testosterone . By reducing the amount of estrogen in the body, Anastrozole slows the growth of tumors that require estrogen to grow .
Cellular Effects
Anastrozole has a significant impact on various types of cells and cellular processes. It reduces the risk of early breast cancer recurrence and controls breast cancer that has come back or spread to other parts of the body . Anastrozole can also reduce the risk of breast cancer development if a person’s family history or a genetic test shows they have a higher risk of breast cancer .
Molecular Mechanism
Anastrozole exerts its effects at the molecular level by blocking the production of estrogens in the body, hence having antiestrogenic effects . It does this by reversibly binding to the aromatase enzyme, and through competitive inhibition, blocks the conversion of androgens to estrogens in peripheral (extragonadal) tissues .
Temporal Effects in Laboratory Settings
Anastrozole is generally safe to take for a long time . It can make bones weaker and more likely to break (osteoporosis). Bone density (DEXA) scans may be conducted to check the strength of the bones before starting Anastrozole treatment, 1 or 2 years into treatment, and again after the treatment finishes .
Dosage Effects in Animal Models
Based on findings from animal studies and its mechanism of action, Anastrozole can cause fetal harm when administered to a pregnant woman . Anastrozole caused embryo-fetal toxicities in rats at maternal exposure that were 9 times the human clinical exposure, based on area under the curve (AUC) .
Metabolic Pathways
Anastrozole is primarily metabolized in the liver via oxidation and glucuronidation to a number of inactive metabolites, including hydroxyanastrozole (both free and glucuronidated) and anastrozole glucuronide . Oxidation to hydroxyanastrozole is catalyzed predominantly by CYP3A4 (as well as CYP3A5 and CYP2C8, to a lesser extent) and the direct glucuronidation of anastrozole is catalyzed mainly by UGT1A4 .
Transport and Distribution
Anastrozole is rapidly absorbed and T max is typically reached within 2 hours of dosing under fasted conditions . Once absorbed, Anastrozole is widely distributed throughout the body, with about 40% being bound to plasma proteins .
Subcellular Localization
The subcellular localization of Anastrozole is not explicitly mentioned in the literature. Given its mechanism of action, it can be inferred that Anastrozole likely interacts with the aromatase enzyme in the endoplasmic reticulum of cells, where the enzyme is located. This is because aromatase is a membrane-bound enzyme, and its active site is located in the endoplasmic reticulum .
Vorbereitungsmethoden
Synthetic Routes: Anastrozole is synthesized through a multistep process. The key step involves the coupling of 1,2,4-triazole with a phenylmethyl group, followed by nitrile addition. The overall reaction sequence leads to the formation of the compound.
Industrial Production: Anastrozole is produced industrially using efficient synthetic routes.
Analyse Chemischer Reaktionen
Reaktivität: Anastrozol unterliegt aufgrund seiner strukturellen Eigenschaften verschiedenen chemischen Reaktionen. Dazu gehören Oxidations-, Reduktions- und Substitutionsreaktionen.
Häufige Reagenzien und Bedingungen: Spezifische Reagenzien und Bedingungen hängen von der gewünschten Transformation ab. Zum Beispiel
Vergleich Mit ähnlichen Verbindungen
Eigenschaften
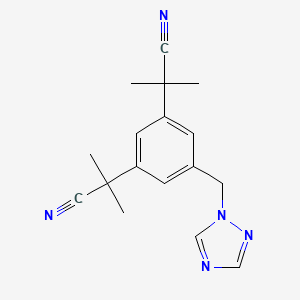
IUPAC Name |
2-[3-(2-cyanopropan-2-yl)-5-(1,2,4-triazol-1-ylmethyl)phenyl]-2-methylpropanenitrile | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
YBBLVLTVTVSKRW-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)(C#N)C1=CC(=CC(=C1)CN2C=NC=N2)C(C)(C)C#N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H19N5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID9022607 | |
| Record name | Anastrozole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022607 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
293.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Anastrozole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015348 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Freely soluble in methanol, acetone, ethanol, tetrahydrofuran; very soluble in acetonitrile., In water, 0.5 mg/mL at 25 °C; solubility is dependent of pH in the physiological range., 6.61e-02 g/L | |
| Record name | Anastrozole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01217 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ANASTROZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7462 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Anastrozole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015348 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Anastrazole exerts its anti-estrogenic effects via selective and competitive inhibition of the aromatase enzyme found predominantly in the adrenal glands, liver, and fatty tissues. Many breast cancers are hormone receptor-positive, meaning their growth is stimulated and/or maintained by the presence of hormones such as estrogen or progesterone. In postmenopausal women, estrogen is primarily derived from the conversion of adrenally-produced androgens into estrogens by the aromatase enzyme - by competitively inhibiting the biosynthesis of estrogen at these enzymes, anastrozole effectively suppresses circulating estrogen levels and, subsequently, the growth of hormone receptor-positive tumours., Anastrozole is a nonsteroidal aromatase inhibitor that interferes with estradiol production in peripheral tissues. Adrenally generated androstenedione, the chief source of circulating estrogen in postmenopausal women, is converted by aromatase to estrone, which is further converted to estradiol. Growth of many breast cancer tumors containing estrogen receptors and aromatase can be promoted by estrogen., Anastrozole is a potent and selective non-steroidal aromatase inhibitor. It significantly lowers serum estradiol concentrations and has no detectable effect on formation of adrenal corticosteroids or aldosterone., Because estrogen acts as a growth factor for hormone-dependent breast cancer cells, anastrozole-induced reduction of serum and tumor concentrations of estrogen inhibits tumor growth and delays disease progression. Anastrozole selectively inhibits the conversion of androgens to estrogens. In postmenopausal women, ovarian secretion of estrogen declines and conversion of adrenal androgens (mainly androstenedione and testosterone) to estrone and estradiol in peripheral tissues (adipose, muscle, and liver), catalyzed by the aromatase enzyme, is the principal source of estrogens. Anastrozole inhibits the aromatase enzyme by competitively binding to the heme of the cytochrome P-450 unit of the enzyme; suppression of estrogen biosynthesis in all tissues reduces serum concentrations of circulating estrogens, including estrone, estradiol, and estrone sulfate. Anastrozole selectively inhibits synthesis of estrogens and does not affect synthesis of adrenal corticosteroid, aldosterone, or thyroid hormone. In animals, anastrozole has not been shown to possess direct progestogenic, androgenic, or estrogenic activity, but alterations in the circulating concentrations of progesterone, androgens, and estrogens have been observed. | |
| Record name | Anastrozole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01217 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ANASTROZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7462 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from ethyl acetate/cyclohexane, Off-white powder | |
CAS No. |
120511-73-1 | |
| Record name | Anastrozole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=120511-73-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Anastrozole [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0120511731 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Anastrozole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01217 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | anastrozole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759855 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | anastrozole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=719344 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Anastrozole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022607 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1,3-Benzenediacetonitrile, α1,α1,α3,α3-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.129.723 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ANASTROZOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/2Z07MYW1AZ | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | ANASTROZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7462 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Anastrozole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015348 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
81-82 °C, 130.14 °C | |
| Record name | ANASTROZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7462 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Anastrozole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015348 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.
![3-amino-5-[(Z)-1-cyano-2-(3,4-dihydroxyphenyl)ethenyl]-1H-pyrazole-4-carbonitrile](/img/structure/B1683694.png)
![(E)-3-[3-(1,3-benzothiazol-2-ylsulfanylmethyl)-4-hydroxy-5-methoxyphenyl]-2-cyanoprop-2-enamide](/img/structure/B1683695.png)
![Benzoic acid, 4-[[(2,5-dihydroxyphenyl)methyl]amino]-, methyl ester](/img/structure/B1683696.png)