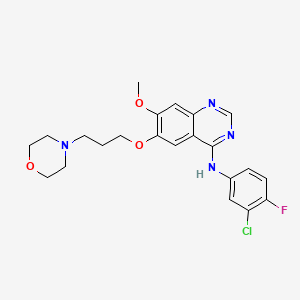
Gefitinib
Übersicht
Beschreibung
Gefitinib, sold under the brand name Iressa, is a medication primarily used for the treatment of certain types of cancer, including non-small cell lung cancer and breast cancer. It is a selective inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase, which plays a crucial role in the regulation of cell growth and survival .
Wissenschaftliche Forschungsanwendungen
Gefitinib hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Als Modellverbindung für die Untersuchung von EGFR-Inhibitoren und deren Wechselwirkungen mit Zielproteinen.
Biologie: Untersucht auf seine Auswirkungen auf Zellsignalwege und Apoptose.
Medizin: Umfassend in klinischen Studien zur Behandlung von nicht-kleinzelligem Lungenkrebs und anderen EGFR-mutierten Krebserkrankungen eingesetzt.
Industrie: Eingesetzt bei der Entwicklung von zielgerichteten Krebstherapien und personalisierten Medizinansätzen
5. Wirkmechanismus
This compound übt seine Wirkung durch Hemmung der EGFR-Tyrosinkinase aus. Es bindet an die Adenosintriphosphat (ATP)-Bindungsstelle des Enzyms und verhindert die Phosphorylierung und Aktivierung von nachgeschalteten Signalwegen. Diese Hemmung führt zur Unterdrückung der Zellproliferation und Induktion der Apoptose in Krebszellen mit überexprimiertem oder mutiertem EGFR .
Wirkmechanismus
Target of Action
Gefitinib, marketed under the trade name Iressa, is a selective inhibitor of the epidermal growth factor receptor’s (EGFR) tyrosine kinase domain . EGFR is a member of a family of receptors (ErbB) which includes Her1 (EGFR), Her2 (erb-B2), Her3 (erb-B3), and Her4 (Erb-B4) . EGFR is often overexpressed in certain types of human carcinomas, such as lung and breast cancer cells .
Mode of Action
This compound inhibits the intracellular phosphorylation of numerous tyrosine kinases associated with transmembrane cell surface receptors, including the tyrosine kinases associated with EGFR . It binds to the adenosine triphosphate (ATP)-binding site of the enzyme . By inhibiting EGFR tyrosine kinase, the downstream signaling cascades are also inhibited, resulting in inhibited malignant cell proliferation .
Biochemical Pathways
This compound selectively targets the mutant proteins in malignant cells . Research on this compound-sensitive non-small cell lung cancers has shown that a mutation in the EGFR tyrosine kinase domain is responsible for activating anti-apoptotic pathways . These mutations tend to confer increased sensitivity to tyrosine kinase inhibitors such as this compound .
Pharmacokinetics
This compound is extensively metabolized in the liver by cytochrome P450s, primarily by CYP3A4, but CYP3A5 and CYP2D6 also play minor roles in this compound metabolism . It is predominantly excreted via the feces, with renal elimination accounting for less than 4% of the administered dose . The bioavailability of this compound is 59% when taken orally . The concentrations of this compound and its major metabolite O-desmethyl this compound in plasma were measured by high-performance liquid chromatography .
Result of Action
This compound interrupts signaling through the EGFR in target cells . Therefore, it is only effective in cancers with mutated and overactive EGFR . It results in robust host-directed immunity against Salmonella infection through proteo-metabolomic reprogramming . It also induces apoptosis in non-small cell lung cancer cells .
Action Environment
The Predicted Environmental Concentration (PEC) / Predicted No Effect Concentration (PNEC) ratio is 1.05x10-3, which means that the use of this compound is predicted to present an insignificant risk to the environment .
Biochemische Analyse
Biochemical Properties
Gefitinib plays a significant role in biochemical reactions. It interacts with various enzymes, proteins, and other biomolecules. This compound is an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase that binds to the adenosine triphosphate (ATP)-binding site of the enzyme . EGFR is often overexpressed in certain human carcinoma cells, such as lung and breast cancer cells .
Cellular Effects
This compound has profound effects on various types of cells and cellular processes. It influences cell function, including impacts on cell signaling pathways, gene expression, and cellular metabolism. In vitro cytotoxicity studies revealed that this compound enhanced the inhibition of cell proliferation and apoptosis in A549 and H1299 cells compared to free this compound .
Molecular Mechanism
This compound exerts its effects at the molecular level through several mechanisms. It binds to the ATP-binding site of the EGFR tyrosine kinase enzyme, inhibiting its activity . This interaction leads to changes in gene expression and cellular functions .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Gefitinib is synthesized through a multi-step process starting from 4,5-dimethoxy-2-nitrobenzoic acid. The synthesis involves several key steps, including demethylation, esterification, side-chain alkylation, reduction, cyclohexylamine formation, chlorination, and ammonia substitution .
Industrial Production Methods: In industrial settings, this compound is produced using optimized synthetic routes to ensure high yield and purity. The process involves strict control of reaction conditions, such as temperature, pH, and solvent selection, to achieve the desired product quality .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Gefitinib durchläuft verschiedene chemische Reaktionen, darunter:
Oxidation: this compound kann zu verschiedenen Metaboliten oxidiert werden.
Reduktion: Reduktionsreaktionen können die Chinolinringstruktur verändern.
Substitution: Substitutionsreaktionen können am aromatischen Ring auftreten und zur Bildung von Derivaten führen.
Häufige Reagenzien und Bedingungen:
Oxidation: Häufige Oxidationsmittel sind Wasserstoffperoxid und Kaliumpermanganat.
Reduktion: Reduktionsmittel wie Natriumborhydrid und Lithiumaluminiumhydrid werden verwendet.
Substitution: Halogenierungsmittel wie Chlor und Brom werden häufig verwendet.
Hauptprodukte: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, umfassen verschiedene this compound-Derivate mit veränderten pharmakologischen Eigenschaften .
Vergleich Mit ähnlichen Verbindungen
Gefitinib wird oft mit anderen EGFR-Inhibitoren verglichen, wie z. B. Erlotinib und Afatinib. Während alle drei Verbindungen auf die EGFR-Tyrosinkinase abzielen, unterscheiden sie sich in ihren pharmakokinetischen Eigenschaften und ihrer klinischen Wirksamkeit:
Erlotinib: Ähnlicher Wirkmechanismus, aber ein anderes Nebenwirkungsprofil und ein anderes Dosierungsschema.
Afatinib: Irreversibler Inhibitor von EGFR, der ein breiteres Wirkungsspektrum gegen EGFR-Mutationen bietet
Einzigartigkeit: this compound ist einzigartig in seiner selektiven Hemmung der EGFR-Tyrosinkinase und seiner Fähigkeit, spezifische Mutationen in Krebszellen anzugreifen, was es zu einem wertvollen Werkzeug in der personalisierten Krebstherapie macht .
Ähnliche Verbindungen:
- Erlotinib
- Afatinib
- Lapatinib
- Osimertinib
Eigenschaften
IUPAC Name |
N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XGALLCVXEZPNRQ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C(C=C2C(=C1)N=CN=C2NC3=CC(=C(C=C3)F)Cl)OCCCN4CCOCC4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H24ClFN4O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8041034 | |
| Record name | Gefitinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8041034 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
446.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Gefitinib | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014462 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Sparingly soluble (|
|
|
| Record name | Gefitinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00317 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Gefitinib | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014462 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Gefitinib is an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase that binds to the adenosine triphosphate (ATP)-binding site of the enzyme. EGFR is often shown to be overexpressed in certain human carcinoma cells, such as lung and breast cancer cells. Overexpression leads to enhanced activation of the anti-apoptotic Ras signal transduction cascades, subsequently resulting in increased survival of cancer cells and uncontrolled cell proliferation. Gefitinib is the first selective inhibitor of the EGFR tyrosine kinase which is also referred to as Her1 or ErbB-1. By inhibiting EGFR tyrosine kinase, the downstream signaling cascades are also inhibited, resulting in inhibited malignant cell proliferation. | |
| Record name | Gefitinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00317 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
184475-35-2 | |
| Record name | Gefitinib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=184475-35-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Gefitinib [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0184475352 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Gefitinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00317 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Gefitinib | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759856 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Gefitinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8041034 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Gefitinib | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | GEFITINIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/S65743JHBS | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Gefitinib | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014462 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details







Synthesis routes and methods II
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.
![(Z)-7-[(1R,2R,3R,5R)-5-chloro-2-[(E,3S)-3-cyclohexyl-3-hydroxyprop-1-enyl]-3-hydroxycyclopentyl]hept-5-enoic acid](/img/structure/B1684392.png)
![(5E)-5-[2-[(1E,3E)-5-hydroxy-5-[1-(3-phenylprop-2-ynyl)cyclobutyl]penta-1,3-dienyl]cyclohexylidene]pentanoic acid](/img/structure/B1684394.png)
![2,3-Dimethyl-6-phenyl-12h-[1,3]dioxolo[4,5-h]imidazo[1,2-c][2,3]benzodiazepine](/img/structure/B1684396.png)
![ethyl N-[4-(2,4-dimethoxyphenyl)-5-oxodithiolo[4,3-b]pyrrol-6-yl]carbamate](/img/structure/B1684403.png)
![4-(4-Chloroanilino)-2-[4-[(2E)-2-hydroxyiminopropanoyl]piperazin-1-yl]-6-propan-2-yl-5H-pyrrolo[3,4-d]pyrimidin-7-one](/img/structure/B1684410.png)
![N-[(2S)-2-(3,4-dichlorophenyl)-4-[4-(2-oxo-1,3-diazinan-1-yl)piperidin-1-yl]butyl]-N-methylbenzamide](/img/structure/B1684412.png)
![3-[benzyl(propan-2-yl)amino]-1-naphthalen-2-ylpropan-1-one;hydrochloride](/img/structure/B1684414.png)
