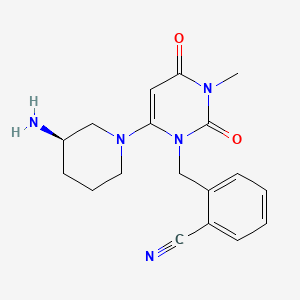
Alogliptin
Overview
Description
Alogliptin is an oral anti-diabetic drug belonging to the dipeptidyl peptidase-4 (DPP-4) inhibitor class. It is primarily used to manage hyperglycemia in patients with type 2 diabetes mellitus. This compound works by inhibiting the DPP-4 enzyme, which normally degrades incretin hormones such as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). By inhibiting DPP-4, this compound increases the levels of active incretin hormones, thereby enhancing insulin secretion and reducing glucagon levels in a glucose-dependent manner .
Mechanism of Action
Target of Action
Alogliptin is a selective, orally-bioavailable inhibitor of the enzymatic activity of dipeptidyl peptidase-4 (DPP-4) . DPP-4 is an enzyme that degrades the incretins glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) . Incretins are hormones that regulate glucose homeostasis by increasing insulin synthesis and release from pancreatic beta cells .
Mode of Action
This compound inhibits DPP-4, which normally degrades the incretins GIP and GLP-1 . The inhibition of DPP-4 increases the amount of active plasma incretins, which helps with glycemic control . This results in increased insulin secretion, increased tissue uptake of glucose, and reduced blood sugar .
Biochemical Pathways
The inhibition of DPP-4 by this compound affects the incretin system, leading to increased levels of active incretins . This results in increased insulin synthesis and release from pancreatic beta cells and decreased glucagon secretion from pancreatic alpha cells . Decreased glucagon secretion results in decreased hepatic glucose production . This compound also impacts the PI3K/Akt/FoxO1 pathway and downstream inflammatory cascades .
Pharmacokinetics
This compound is rapidly absorbed (median Tmax, 1-2 hours) and eliminated slowly (mean t1/2, 12.4-21.4 hours), primarily via urinary excretion (mean fraction of drug excreted in urine from 0 to 72 hours after dosing, 60%-71%) . Cmax and AUC0-infinity increased dose proportionally over the range from 25 to 100 mg .
Result of Action
The molecular effects of this compound include decreased mitochondrial reactive oxygen species production rate, prevention of mitochondrial membrane depolarization, and alleviation of mitochondrial swelling in diabetic rabbits . It also improves mitochondrial biogenesis by peroxisome proliferator-activated receptor-coactivator 1/nuclear respiratory factor-1/mitochondrial transcription factor A signaling regulated by adiponectin/AMP-activated protein kinase . The cellular effects include left ventricular hypertrophy and left atrial dilation without obvious hemodynamic abnormalities, and these changes were attenuated by this compound .
Action Environment
The action of this compound can be influenced by environmental factors such as diet and exercise . It is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus . The efficacy and stability of this compound can also be affected by the patient’s renal function, as AUC increased two-, three-, and four-fold in patients with moderate renal impairment, severe renal impairment, and end-stage renal disease, respectively .
Biochemical Analysis
Biochemical Properties
Alogliptin interacts with the enzyme dipeptidyl peptidase-4 (DPP-4). This enzyme normally degrades the incretins glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). This compound inhibits DPP-4, thereby increasing the levels of these incretins .
Cellular Effects
This compound has been shown to have effects on various types of cells and cellular processes. It influences cell function by increasing the levels of incretins, which in turn help the body produce more insulin and stop the body from releasing too much sugar into the blood . This helps keep blood sugar levels stable.
Molecular Mechanism
The molecular mechanism of action of this compound involves the inhibition of the DPP-4 enzyme. This inhibition results in prolonged active incretin levels, which help the body produce more insulin and stop the body from releasing too much sugar into the blood .
Temporal Effects in Laboratory Settings
In laboratory settings, peak inhibition of DPP-4 occurs within 2-3 hours after a single-dose administration of this compound to healthy subjects. The peak inhibition of DPP-4 exceeded 93% across doses of 12.5 mg to 800 mg. Inhibition of DPP-4 remained above 80% at 24 hours for doses greater than or equal to 25 mg .
Dosage Effects in Animal Models
While specific studies on the dosage effects of this compound in animal models were not found, it is generally recommended that the dosage of this compound be adjusted based on the patient’s kidney function .
Metabolic Pathways
This compound is involved in the metabolic pathway related to the regulation of blood sugar levels. It interacts with the DPP-4 enzyme and incretins such as GIP and GLP-1 .
Transport and Distribution
This compound is well distributed into tissues. Following a single, 12.5 mg intravenous infusion of this compound to healthy subjects, the volume of distribution during the terminal phase was 417 L .
Subcellular Localization
As a small molecule drug, this compound is likely to be distributed throughout the cell, where it can interact with its target, the DPP-4 enzyme .
Preparation Methods
Synthetic Routes and Reaction Conditions
Alogliptin is synthesized through a multi-step process involving the formation of key intermediates. The synthetic route typically starts with the preparation of 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile. This intermediate is then subjected to various chemical reactions, including amination and cyclization, to yield the final product .
Industrial Production Methods
In industrial settings, the production of this compound involves the use of high-performance liquid chromatography (HPLC) for purification and quality control. The process is optimized to ensure high yield and purity of the final product. The use of advanced analytical techniques, such as reverse-phase HPLC (RP-HPLC), is common in the industrial production of this compound .
Chemical Reactions Analysis
Types of Reactions
Alogliptin undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions to form corresponding oxides.
Reduction: Reduction reactions can be used to modify the functional groups in this compound.
Substitution: This compound can undergo substitution reactions, where specific atoms or groups are replaced by others
Common Reagents and Conditions
Common reagents used in the chemical reactions of this compound include:
Oxidizing agents: Such as hydrogen peroxide and potassium permanganate.
Reducing agents: Such as sodium borohydride and lithium aluminum hydride.
Substituting agents: Such as halogens and alkylating agents
Major Products Formed
The major products formed from the chemical reactions of this compound depend on the specific reaction conditions and reagents used. For example, oxidation may yield oxides, while substitution reactions may result in halogenated or alkylated derivatives .
Scientific Research Applications
Alogliptin has a wide range of scientific research applications, including:
Comparison with Similar Compounds
Alogliptin is part of the DPP-4 inhibitor class, which includes other similar compounds such as:
- Sitagliptin
- Saxagliptin
- Linagliptin
- Vildagliptin
Uniqueness of this compound
This compound is unique among DPP-4 inhibitors due to its high selectivity and oral bioavailability. It has a relatively long half-life, allowing for once-daily dosing. Additionally, this compound has been shown to have a favorable safety profile, with a low risk of hypoglycemia and minimal impact on body weight .
Properties
IUPAC Name |
2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]benzonitrile | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H21N5O2/c1-21-17(24)9-16(22-8-4-7-15(20)12-22)23(18(21)25)11-14-6-3-2-5-13(14)10-19/h2-3,5-6,9,15H,4,7-8,11-12,20H2,1H3/t15-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZSBOMTDTBDDKMP-OAHLLOKOSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1C(=O)C=C(N(C1=O)CC2=CC=CC=C2C#N)N3CCCC(C3)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN1C(=O)C=C(N(C1=O)CC2=CC=CC=C2C#N)N3CCC[C@H](C3)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H21N5O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID90234130 | |
| Record name | Alogliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90234130 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
339.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Sparingly soluble | |
| Record name | Alogliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06203 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Alogliptin inhibits dipeptidyl peptidase 4 (DPP-4), which normally degrades the incretins glucose-dependent insulinotropic polypeptide (GIP) and glucagon like peptide 1 ( GLP-1). The inhibition of DPP-4 increases the amount of active plasma incretins which helps with glycemic control. GIP and GLP-1 stimulate glucose dependent secretion of insulin in pancreatic beta cells. GLP-1 has the additional effects of suppressing glucose dependent glucagon secretion, inducing satiety, reducing food intake, and reducing gastric emptying., Increased concentrations of the incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the DPP-4 enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. In patients with type 2 diabetes, concentrations of GLP-1 are reduced but the insulin response to GLP-1 is preserved. Alogliptin is a DPP-4 inhibitor that slows the inactivation of the incretin hormones, thereby increasing their bloodstream concentrations and reducing fasting and postprandial glucose concentrations in a glucose-dependent manner in patients with type 2 diabetes mellitus. Alogliptin selectively binds to and inhibits DPP-4 but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures. | |
| Record name | Alogliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06203 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Alogliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8203 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
850649-61-5 | |
| Record name | Alogliptin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=850649-61-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Alogliptin [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0850649615 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Alogliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06203 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Alogliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90234130 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]benzonitrile | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ALOGLIPTIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/JHC049LO86 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Alogliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8203 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: [, , , , , , , , ] Alogliptin functions as a highly selective dipeptidyl peptidase-4 (DPP-4) inhibitor. DPP-4 is an enzyme that rapidly inactivates incretin hormones, primarily glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). By inhibiting DPP-4, this compound increases the levels of GLP-1 and GIP, which in turn leads to enhanced insulin secretion from pancreatic beta cells and suppressed glucagon secretion, ultimately resulting in improved glucose homeostasis.
A: The molecular formula of this compound benzoate (the active form) is C18H18N6O3 • C7H6O2, and its molecular weight is 462.46 g/mol.
A: While the provided papers don’t delve into detailed formulation strategies for this compound, one study explored the use of injectable long-acting poly (lactide-co-glycolide) (PLGA)-based in situ gel implants (ISGI) loaded with this compound . This approach aimed to provide sustained therapeutic exposures and improve pharmacological responses, potentially leading to reduced dosing frequency and enhanced patient compliance. The study highlighted the effectiveness of solvents like N-methyl-2-pyrrolidone (NMP) and dimethyl sulfoxide (DMSO) in achieving successful ISGI preparation and controlling the release profile of this compound.
A: [, , ] this compound demonstrates moderate absorption, exceeding 75%, and its absorption is not significantly affected by food [, ]. It exhibits slow-binding properties to the DPP-4 enzyme, resulting in sustained reduction of plasma DPP-4 activity . This compound has low plasma protein binding . Although the provided papers don't extensively detail its metabolism, they indicate that this compound is primarily excreted renally .
A: Research indicates that creatinine clearance and weight can significantly influence the oral clearance (CL/F) of this compound . This suggests that dose adjustments may be necessary for patients with renal impairment, while weight-based adjustments are generally not clinically relevant.
A: [, ] Studies haven't identified any significant drug interactions with this compound monotherapy [, ]. Notably, it can be co-administered with medications like ketoconazole, fluconazole, gemfibrozil, warfarin, metformin, glyburide, and pioglitazone without requiring dosage adjustments .
A: A study using diabetic apolipoprotein E-deficient mice showed that this compound treatment effectively reduced atherosclerotic lesions . This protective effect was linked to this compound's ability to lower plasma glucose levels and attenuate the expression of inflammatory cytokines (IL-6 and IL-1β) within the atherosclerotic plaques.
A: Research in rabbits suggests that this compound exhibits cardioprotective effects against ischemia-reperfusion injury . The study attributed these benefits to this compound's ability to enhance nitric oxide production through both GLP-1 receptor-dependent and -independent pathways. This increased nitric oxide production was associated with improved left ventricular ejection fraction and ±dP/dt, indicating enhanced cardiac function.
A: [, , , , , , , , , , ] Numerous clinical trials have demonstrated the efficacy of this compound in improving glycemic control in patients with type 2 diabetes. This compound, as monotherapy or in combination with other antidiabetic agents, consistently led to significant reductions in HbA1c levels [, , , , , , , , , , ]. Studies also highlight its efficacy in specific patient populations, such as those with inadequate glycemic control on metformin [, , ] or those receiving insulin therapy .
A: While traditional oral formulations of this compound are available, researchers are investigating injectable long-acting PLGA-based ISGI as a potential alternative delivery system. This approach aims to enhance patient compliance and potentially improve the therapeutic outcomes by achieving sustained drug release.
A: Research suggests that urinary angiotensinogen (AGT) levels could potentially serve as a prognostic marker for the renoprotective effects of this compound in patients with type 2 diabetes . The study observed a correlation between higher baseline urinary AGT levels and a more pronounced decrease in urinary albumin-to-creatinine ratio (UACR) following this compound treatment. This finding implies that urinary AGT could potentially help identify patients who are more likely to benefit from the renoprotective effects of this compound.
A: [, , ] Several analytical methods have been developed and validated for the quantification of this compound, including:
- UV Spectrophotometry: This technique utilizes the absorbance of UV-visible light by this compound at specific wavelengths for its quantification. It's a simple and cost-effective method often used for routine analysis of this compound in pharmaceutical formulations. [, ]
- High-Performance Thin-Layer Chromatography (HPTLC): HPTLC is a versatile technique that separates and quantifies this compound based on its differential migration on a thin layer of adsorbent material.
- Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC): RP-HPLC offers high sensitivity and selectivity in separating and quantifying this compound in complex matrices like pharmaceutical formulations and biological samples. It's often coupled with UV detection for quantification. [, ]
- Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS): LC-MS/MS provides exceptional sensitivity and specificity for quantifying this compound in biological matrices, particularly at low concentrations. It's often the preferred method for pharmacokinetic studies.
A: [, , ] The development and validation of analytical methods for this compound quantification involve assessing key validation parameters as outlined by regulatory guidelines, such as the International Conference on Harmonisation (ICH) guidelines. These parameters include:
A: [, , , , ] Several alternatives to this compound are available for managing type 2 diabetes, including:
- Other DPP-4 Inhibitors: As mentioned earlier, other DPP-4 inhibitors like sitagliptin, vildagliptin, saxagliptin, and linagliptin offer similar mechanisms of action and comparable efficacy to this compound in terms of HbA1c reduction. [, , , , ]
- Metformin: Metformin is a first-line treatment for type 2 diabetes that improves insulin sensitivity and reduces hepatic glucose production. It is often used in combination with DPP-4 inhibitors or GLP-1 receptor agonists. [, ]
A: [, ] this compound received approval from the US Food and Drug Administration (FDA) for the treatment of type 2 diabetes in 2013. [, ]
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


![[5-(3,6-diaminohexanoylamino)-3-hydroxy-2-(hydroxymethyl)-6-[(5-methyl-4-oxo-3a,6,7,7a-tetrahydro-1H-imidazo[4,5-c]pyridin-2-yl)amino]oxan-4-yl] carbamate](/img/structure/B1666815.png)