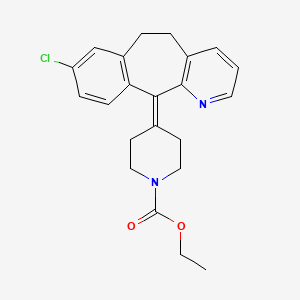
Loratadina
Descripción general
Descripción
La loratadina es un antihistamínico de segunda generación ampliamente utilizado para controlar los síntomas de la rinitis alérgica y la urticaria. Es conocida por su efectividad en el tratamiento de los síntomas de alergia como estornudos, picazón y ojos llorosos sin causar sedación significativa, lo que la hace preferible a los antihistamínicos de primera generación .
Mecanismo De Acción
La loratadina actúa como un agonista inverso selectivo para los receptores periféricos de histamina H1. Cuando la histamina se libera durante una reacción alérgica, se une a estos receptores, causando síntomas como picazón y estornudos. La this compound bloquea esta unión, deteniendo eficazmente la respuesta alérgica . Tiene efectos mínimos en el sistema nervioso central, lo que reduce el riesgo de sedación .
Aplicaciones Científicas De Investigación
La loratadina tiene una amplia gama de aplicaciones de investigación científica:
Análisis Bioquímico
Biochemical Properties
Loratadine functions primarily by binding to histamine H1 receptors, which are G-protein coupled receptors located on the surface of various cells, including epithelial cells, endothelial cells, eosinophils, neutrophils, airway cells, and vascular smooth muscle cells . By binding to these receptors, loratadine prevents histamine from exerting its effects, thereby reducing allergic symptoms. The interaction between loratadine and the H1 receptor is characterized by its high affinity and selectivity, which contributes to its effectiveness in blocking histamine-induced responses .
Cellular Effects
Loratadine exerts several effects on different cell types and cellular processes. It has been shown to influence cell signaling pathways, gene expression, and cellular metabolism. For instance, loratadine can modulate the activity of various signaling molecules involved in inflammatory responses, such as cytokines and chemokines . Additionally, loratadine has been associated with improved prognosis in certain cancers, such as lung cancer, by inducing apoptosis and reducing epithelial-mesenchymal transition . These effects are mediated through its interaction with specific cellular receptors and signaling pathways, highlighting its potential therapeutic benefits beyond allergy management.
Molecular Mechanism
At the molecular level, loratadine exerts its effects by acting as an inverse agonist of the histamine H1 receptor . This means that loratadine not only blocks the binding of histamine to the receptor but also stabilizes the receptor in its inactive state, thereby reducing its basal activity. The binding of loratadine to the H1 receptor involves interactions with specific amino acid residues within the receptor’s binding pocket, which prevents the conformational changes required for receptor activation . This mechanism of action underlies the antihistaminic and anti-inflammatory effects of loratadine.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of loratadine have been studied over various time periods to assess its stability, degradation, and long-term impact on cellular function. Loratadine is known to have a relatively long half-life, which contributes to its sustained therapeutic effects . Studies have shown that loratadine and its active metabolite, desloratadine, can maintain their efficacy over extended periods, with minimal degradation . Additionally, long-term exposure to loratadine has been associated with consistent anti-inflammatory and antihistaminic effects, further supporting its use in chronic allergic conditions .
Dosage Effects in Animal Models
The effects of loratadine at different dosages have been extensively studied in animal models. These studies have revealed that loratadine exhibits dose-dependent effects, with higher doses leading to more pronounced therapeutic outcomes . Excessive dosages can result in adverse effects, such as drowsiness, dry mouth, and gastrointestinal disturbances . In cancer models, moderate concentrations of loratadine have been shown to induce apoptosis and reduce epithelial-mesenchymal transition, while higher concentrations can trigger pyroptosis . These findings highlight the importance of optimizing loratadine dosage to achieve the desired therapeutic effects while minimizing potential side effects.
Metabolic Pathways
Loratadine undergoes extensive first-pass metabolism in the liver, primarily mediated by cytochrome P450 enzymes, including CYP3A4, CYP2D6, CYP1A1, and CYP2C19 . The major metabolite of loratadine is desloratadine, which retains antihistaminic activity and contributes to the overall therapeutic effects of the drug . The metabolic pathways involved in loratadine metabolism also include hydroxylation and conjugation reactions, which facilitate the elimination of the drug from the body . Understanding these metabolic pathways is crucial for optimizing loratadine dosing and minimizing potential drug interactions.
Transport and Distribution
Loratadine is transported and distributed within cells and tissues through various mechanisms. It binds to plasma proteins, which facilitates its distribution throughout the body . The tissue distribution of loratadine and its metabolites has been studied in animal models, revealing that they are widely distributed in organs such as the liver, spleen, thymus, heart, adrenal glands, and pituitary gland . The concentrations of loratadine and its metabolites in these tissues are influenced by factors such as blood flow, tissue permeability, and binding affinity to cellular receptors . These findings provide insights into the pharmacokinetics and tissue-specific effects of loratadine.
Subcellular Localization
The subcellular localization of loratadine and its effects on cellular activity have been investigated to understand its precise mechanism of action. Loratadine is known to localize to specific cellular compartments, such as the plasma membrane and cytoplasm, where it interacts with histamine H1 receptors . The targeting of loratadine to these compartments is facilitated by its chemical structure and binding properties, which enable it to effectively block histamine-induced signaling pathways . Additionally, loratadine may undergo post-translational modifications that influence its localization and activity within cells
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La loratadina se puede sintetizar a través de varios métodos. Un método común implica la reacción del 4-(8-cloro-5,6-dihidro-11H-benzo[5,6]ciclohepta[1,2-b]piridin-11-ilideno)-1-piperidinocarboxilato de etilo con reactivos apropiados en condiciones controladas. El proceso generalmente incluye pasos como disolver la this compound cruda en un solvente orgánico, agregar carbón activado para adsorción, calentar, agitar y filtrar para obtener this compound purificada .
Métodos de producción industrial: En entornos industriales, la this compound se produce utilizando homogeneización de alta presión de cizallamiento de alta velocidad seguida de liofilización para crear nanocristales de this compound. Este método mejora la solubilidad y la biodisponibilidad de la this compound, lo que la hace más efectiva para la administración oral .
Análisis De Reacciones Químicas
Tipos de reacciones: La loratadina experimenta varias reacciones químicas, incluida la oxidación, la reducción y la sustitución. Por ejemplo, es probable que ocurra la oxidación en los anillos de piperidina y cicloheptano .
Reactivos y condiciones comunes: Los reactivos comunes utilizados en estas reacciones incluyen peróxido de hidrógeno para la oxidación y borohidruro de sodio para la reducción. Las condiciones generalmente implican temperaturas controladas y niveles de pH para asegurar los resultados de reacción deseados.
Principales productos formados: Los principales productos formados a partir de estas reacciones incluyen la desthis compound, que es un metabolito activo de la this compound y conserva propiedades antihistamínicas .
Comparación Con Compuestos Similares
La loratadina a menudo se compara con otros antihistamínicos de segunda generación como la cetirizina y la fexofenadina. A diferencia de los antihistamínicos de primera generación como la difenhidramina, la this compound no cruza la barrera hematoencefálica de manera significativa, lo que da como resultado menos efectos sedantes . Compuestos similares incluyen:
Cetirizina: Conocida por su efectividad en el tratamiento de reacciones alérgicas, pero puede causar somnolencia leve.
Fexofenadina: Otro antihistamínico no sedante con un mecanismo de acción similar.
Clorfeniramina: Un antihistamínico de primera generación que es efectivo pero causa sedación significativa.
La ventaja única de la this compound radica en su capacidad de brindar alivio eficaz de las alergias sin causar somnolencia, lo que la convierte en la opción preferida para muchos pacientes.
Propiedades
IUPAC Name |
ethyl 4-(13-chloro-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene)piperidine-1-carboxylate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JCCNYMKQOSZNPW-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC(=O)N1CCC(=C2C3=C(CCC4=C2N=CC=C4)C=C(C=C3)Cl)CC1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H23ClN2O2 | |
| Record name | loratadine | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Loratadine | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2023224 | |
| Record name | Loratadine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2023224 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
382.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Loratadine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005000 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
<1 mg/ml at 25°C | |
| Record name | Loratadine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00455 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Histamine release is a key mediator in allergic rhinitis and urticaria. As a result, loratadine exerts it's effect by targeting H1 histamine receptors. Loratadine binds to H1 histamine receptors found on the surface of epithelial cells, endothelial cells, eosinophils, neutrophils, airway cells, and vascular smooth muscle cells among others. H1 histamine receptors fall under the wider umbrella of G-protein coupled receptors, and exist in a state of equilibrium between the active and inactive forms. Histamine binding to the H1-receptor facilitates cross linking between transmembrane domains III and V, stabilizing the active form of the receptor. On the other hand, antihistamines bind to a different site on the H1 receptor favouring the inactive form. Hence, loratadine can more accurately be classified as an "inverse agonist" as opposed to a "histamine antagonist", and can prevent or reduce the severity of histamine mediated symptoms., All of the available H1 receptor antagonists are reversible, competitive inhibitors of the interaction of histamine with H1 receptors. /H1 Receptor Antagonists/, H1 antagonists inhibit most responses of smooth muscle to histamine. /H1 Antagonists Receptors/, Within the vascular tree, the H1 antagonists inhibit both the vasoconstrictor effects of histamine and, to a degree, the more rapid vasodilator effects that are mediated by H1 receptors on endothelial cells. /H1 Receptor Antagonists/, H1 antagonists strongly block the action of histamine that results in increased capillary permeability and formation of edema and wheal. /H1 Receptor Antagonists/, For more Mechanism of Action (Complete) data for LORATADINE (6 total), please visit the HSDB record page. | |
| Record name | Loratadine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00455 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LORATADINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3578 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from acetonitrile | |
CAS No. |
79794-75-5 | |
| Record name | Loratadine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=79794-75-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Loratadine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0079794755 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Loratadine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00455 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | loratadine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758628 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | loratadine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=721075 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Loratadine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2023224 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine-1-carboxylate | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.216.235 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | 1-Piperidinecarboxylic acid, 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-, ethyl ester | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.120.122 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LORATADINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/7AJO3BO7QN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | LORATADINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3578 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Loratadine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005000 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
134-136 °C, 134 - 136 °C | |
| Record name | Loratadine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00455 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LORATADINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3578 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Loratadine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005000 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details









Synthesis routes and methods II
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.
![N-[(3S,4R,5S,9R,10R,13R,14R,17R)-4-hydroxy-10,13-dimethyl-17-[(2R)-1-(4-propan-2-ylidene-2,3-dihydropyrrol-5-yl)propan-2-yl]-2,3,4,5,6,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]acetamide](/img/structure/B1675032.png)