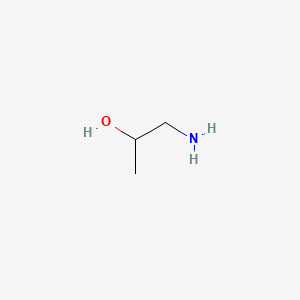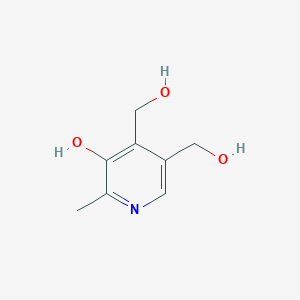
Piridoxina
Descripción general
Descripción
La piridoxina, comúnmente conocida como vitamina B6, es una vitamina soluble en agua que juega un papel crucial en diversas funciones biológicas. Se encuentra naturalmente en muchos alimentos y también está disponible como suplemento dietético. La this compound es esencial para el metabolismo de los aminoácidos, los carbohidratos y los lípidos, y apoya la salud del cerebro, la función inmunitaria y la síntesis de neurotransmisores .
Aplicaciones Científicas De Investigación
La piridoxina tiene una amplia gama de aplicaciones de investigación científica:
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La piridoxina se puede sintetizar mediante varios métodos. Un enfoque común implica la condensación de cianoacetamida con compuestos 1,3-dicarbonílicos. Otro método utiliza la condensación de derivados de 1,3-oxazol con dienófilos, seguida de hidrogenación catalítica . Estos métodos se caracterizan por altos rendimientos y condiciones de reacción suaves.
Métodos de producción industrial: La producción industrial de this compound generalmente implica el método "oxazol", que es un proceso de dos etapas. La primera etapa involucra la condensación de dienos para formar compuestos intermedios, que luego se convierten en this compound mediante hidrogenación catalítica . Este método se usa ampliamente debido a su eficiencia y rentabilidad.
Análisis De Reacciones Químicas
Tipos de reacciones: La piridoxina experimenta varias reacciones químicas, incluida la oxidación, la reducción y la sustitución. Se puede oxidar selectivamente para formar piridoxal o clorhidrato de piridoxal utilizando un sistema de oxidación catalítica .
Reactivos y condiciones comunes: Los reactivos comunes utilizados en la oxidación de la this compound incluyen fuentes de oxígeno, catalizadores, sales inorgánicas y ligandos de amina. Las reacciones generalmente se llevan a cabo en agua como disolvente en condiciones suaves .
Productos principales: Los principales productos formados a partir de la oxidación de la this compound son el piridoxal y el clorhidrato de piridoxal, que son intermediarios clave en la síntesis de piridoxal 5'-fosfato, la forma activa de coenzima de la vitamina B6 .
Mecanismo De Acción
La piridoxina se convierte en piridoxal 5'-fosfato en el cuerpo, que actúa como coenzima en varias reacciones bioquímicas. Está involucrada en el metabolismo de los aminoácidos, el glucógeno y los lípidos, y ayuda en la síntesis de neurotransmisores como la serotonina, la dopamina, la noradrenalina y el ácido gamma-aminobutírico (GABA) . El piridoxal 5'-fosfato también juega un papel en la síntesis de hemoglobina y esfingolípidos .
Comparación Con Compuestos Similares
La piridoxina es parte del grupo de la vitamina B6, que incluye piridoxal y piridoxamina, junto con sus derivados fosforilados. Estos compuestos son químicamente similares y pueden interconvertirse en sistemas biológicos . El piridoxal 5'-fosfato tiene la mayor actividad biológica entre estos compuestos, pero todas las formas son esenciales para varias reacciones enzimáticas .
Compuestos similares:
- Piridoxal
- Piridoxamina
- This compound 5'-fosfato
- Piridoxal 5'-fosfato
- Piridoxamina 5'-fosfato
La this compound es única en su estabilidad y es la forma más utilizada en los suplementos dietéticos .
Propiedades
IUPAC Name |
4,5-bis(hydroxymethyl)-2-methylpyridin-3-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C8H11NO3/c1-5-8(12)7(4-11)6(3-10)2-9-5/h2,10-12H,3-4H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LXNHXLLTXMVWPM-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NC=C(C(=C1O)CO)CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C8H11NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
58-56-0 (hydrochloride) | |
| Record name | Pyridoxine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000065236 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID4023541 | |
| Record name | Pyridoxine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4023541 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
169.18 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
White powder; [Alfa Aesar MSDS], Solid | |
| Record name | Pyridoxine | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/17041 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
| Record name | Pyridoxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000239 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
79 mg/mL | |
| Record name | Pyridoxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00165 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pyridoxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000239 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
0.00000028 [mmHg] | |
| Record name | Pyridoxine | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/17041 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
Mechanism of Action |
Vitamin B6 is the collective term for a group of three related compounds, pyridoxine (PN), pyridoxal (PL) and pyridoxamine (PM), and their phosphorylated derivatives, pyridoxine 5'-phosphate (PNP), pyridoxal 5'-phosphate (PLP) and pyridoxamine 5'-phosphate (PMP). Although all six of these compounds should technically be referred to as vitamin B6, the term vitamin B6 is commonly used interchangeably with just one of them, pyridoxine. Vitamin B6, principally in its biologically active coenzyme form pyridoxal 5'-phosphate, is involved in a wide range of biochemical reactions, including the metabolism of amino acids and glycogen, the synthesis of nucleic acids, hemogloblin, sphingomyelin and other sphingolipids, and the synthesis of the neurotransmitters serotonin, dopamine, norepinephrine and gamma-aminobutyric acid (GABA). | |
| Record name | Pyridoxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00165 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
65-23-6 | |
| Record name | Pyridoxine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=65-23-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pyridoxine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000065236 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Pyridoxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00165 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | pyridoxine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759148 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 3,4-Pyridinedimethanol, 5-hydroxy-6-methyl- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Pyridoxine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4023541 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Pyridoxine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.548 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PYRIDOXINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/KV2JZ1BI6Z | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Pyridoxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000239 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
159-162 °C, 159 - 162 °C | |
| Record name | Pyridoxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00165 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pyridoxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000239 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details











Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
ANone: Pyridoxine is a precursor to pyridoxal 5'-phosphate (PLP), the active form of vitamin B6. PLP functions as a crucial coenzyme for over 100 enzymes involved in various metabolic pathways. [] This includes the metabolism of amino acids, carbohydrates, lipids, neurotransmitters, and heme. [, , ]
ANone: Pyridoxine, through its conversion to PLP, plays a vital role in the biosynthesis of several neurotransmitters, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and histamine. [, , ] It participates in decarboxylation and transamination reactions essential for their production.
ANone: Pyridoxine deficiency can disrupt the synthesis of neurotransmitters. For instance, inadequate pyridoxine levels can lead to reduced GABA synthesis, potentially contributing to seizures. [, ] This highlights the critical role of pyridoxine in maintaining normal neurological function.
ANone: The molecular formula of pyridoxine is C8H11NO3, and its molecular weight is 169.18 g/mol.
ANone: While the provided research papers focus primarily on the biological effects of pyridoxine, several analytical techniques have been employed. UV spectrophotometry is one such method used to quantify pyridoxine in pharmaceutical formulations, utilizing its absorption characteristics. [, , ]
ANone: Pyridoxine, when incorporated into soybean lecithin-based extenders for goat semen, demonstrated beneficial effects on sperm quality after the freeze-thawing process. [] This suggests the compatibility and potential stabilizing properties of pyridoxine in specific biological formulations.
ANone: The study on 4'-deoxypyridoxine provides insights into the structure-activity relationship of pyridoxine. This compound, a pyridoxine analog, demonstrated the ability to both inhibit and stimulate the growth of an Escherichia coli mutant, depending on the concentration of pyridoxal. [] This suggests that even subtle modifications to the pyridoxine structure can significantly alter its biological effects.
ANone: While specific formulation strategies were not discussed in the provided abstracts, research involving pyridoxine often focuses on its administration and bioavailability. For instance, in the treatment of pyridoxine-dependent epilepsy, the timing of pyridoxine supplementation, including antenatal administration, has been explored to optimize its therapeutic efficacy. [] This highlights the ongoing efforts to improve the delivery and effectiveness of pyridoxine in clinical settings.
ANone: Information pertaining to SHE regulations was not covered in the provided research.
ANone: Research suggests that chronic levodopa administration, commonly used in Parkinson's disease, might influence pyridoxine metabolism. [] Patients on chronic levodopa treatment exhibited higher plasma and erythrocyte PLP concentrations after receiving intravenous pyridoxine compared to levodopa-naive controls. [] This finding suggests an adaptive alteration in pyridoxine metabolism induced by levodopa, highlighting the complex interplay between medications and nutrient metabolism.
ANone: Studies using mouse colonic epithelial cells and human colonic apical membrane vesicles revealed that pyridoxine uptake is a carrier-mediated process, suggesting the existence of specific transporters for pyridoxine absorption in the colon. [] Similarly, research on pancreatic acinar cells demonstrated a regulatable and specific carrier-mediated mechanism for pyridoxine uptake, indicating that different cell types may possess unique mechanisms for pyridoxine transport and utilization. []
ANone: Research in rats indicates that pyridoxine might offer protection against the toxic effects of linezolid, an antibiotic. [] Co-administration of pyridoxine with linezolid attenuated hematological toxicity, hepatotoxicity, and oxidative stress markers in rats, suggesting a potential role for pyridoxine in mitigating drug-induced adverse effects. []
ANone: While the exact mechanisms underlying PDE are still being elucidated, research has established a strong link between mutations in the ALDH7A1 gene, responsible for encoding alpha-aminoadipic semialdehyde (AASA) dehydrogenase, and the development of PDE. [, ] These mutations can lead to a deficiency in AASA dehydrogenase activity, resulting in the accumulation of AASA and the development of seizures. [, ] Supplementation with pyridoxine can alleviate seizures in affected individuals, although the specific mechanisms underlying its therapeutic benefits are not fully understood. []
ANone: Research on Jian carp suggests that dietary pyridoxine supplementation can enhance disease resistance and immune responses in fish. [] Fish fed diets containing pyridoxine exhibited higher survival rates after bacterial challenge, along with improvements in various immune parameters. [] These findings highlight the potential of pyridoxine as a dietary supplement for promoting fish health and resilience in aquaculture settings.
ANone: The provided abstracts do not contain information about resistance or cross-resistance to pyridoxine.
ANone: The provided abstracts do not contain information about toxicity or long-term effects of pyridoxine.
ANone: While specific drug delivery strategies for pyridoxine were not discussed in detail, research highlights the importance of its bioavailability and transport. For example, studies using isolated rat liver cells investigated the uptake and metabolism of pyridoxine glucosides. [] This research suggests that the form in which pyridoxine is present in food can affect its absorption and utilization by the body.
ANone: Urinary α-aminoadipic semialdehyde (aAASA) levels have emerged as a potential biomarker for PDE. [] Elevated aAASA levels in urine can indicate a deficiency in AASA dehydrogenase activity, which is the underlying metabolic defect in PDE. [] Monitoring aAASA levels could help clinicians assess the effectiveness of pyridoxine treatment and adjust dosages as needed.
ANone: Researchers utilize various analytical techniques to study pyridoxine. High-performance liquid chromatography (HPLC) coupled with UV detection is one method for quantifying pyridoxine in multivitamin preparations. [, ] This technique allows for the separation and measurement of pyridoxine and other vitamins in complex mixtures.
ANone: Researchers prioritize the validation of analytical methods used in pyridoxine research. A study validating an HPLC method for simultaneous analysis of metamizole, thiamine, and pyridoxine in tablets highlights the importance of accuracy, precision, and specificity in analytical measurements. [] By adhering to strict validation procedures, researchers ensure the quality and reliability of their data, which is crucial for making accurate interpretations and drawing meaningful conclusions.
ANone: The provided research abstracts did not focus on these aspects related to pyridoxine.
ANone: The recognition of pyridoxine as an essential nutrient and the subsequent characterization of pyridoxine deficiency syndromes represent significant milestones. [, ] Early research established the link between pyridoxine deficiency and various conditions, including dermatitis, anemia, and neurological disorders. [, ]
ANone: Pyridoxine research extends beyond the realm of nutrition science and demonstrates significant overlap with other disciplines. For instance, the investigation of pyridoxine-dependent epilepsy involves collaboration between geneticists, neurologists, and biochemists to unravel the complex interplay between genetic mutations, metabolic pathways, and clinical manifestations. [, ]
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.

![Acetyloxymethyl 2-[6-[bis[2-(acetyloxymethoxy)-2-oxoethyl]amino]-5-[2-[6-[bis[2-(acetyloxymethoxy)-2-oxoethyl]amino]-2,3-difluorophenoxy]ethoxy]-1-benzofuran-2-yl]-1,3-oxazole-5-carboxylate](/img/structure/B162723.png)
![3-[(Dimethylamino)methyl]-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one](/img/structure/B162766.png)