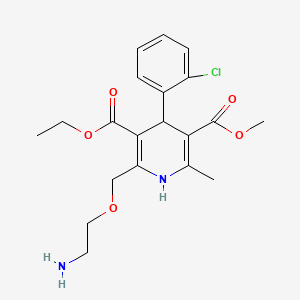
Amlodipino
Descripción general
Descripción
Amlodipino es un bloqueador de los canales de calcio de acción prolongada de la clase de las dihidropiridinas. Se utiliza principalmente para tratar la presión arterial alta y la enfermedad de las arterias coronarias. This compound funciona relajando los vasos sanguíneos, lo que permite que la sangre fluya más fácilmente, lo que ayuda a reducir la presión arterial y reduce la carga de trabajo del corazón .
Mecanismo De Acción
Amlodipino ejerce sus efectos inhibiendo la entrada de iones calcio en las células musculares lisas vasculares y en las células musculares cardíacas. Esta inhibición conduce a la relajación de los vasos sanguíneos, lo que da como resultado una disminución de la resistencia vascular periférica y una presión arterial más baja. Los objetivos moleculares de this compound incluyen los canales de calcio de tipo L, que desempeñan un papel fundamental en la contracción del músculo liso vascular .
Aplicaciones Científicas De Investigación
Amlodipino tiene una amplia gama de aplicaciones de investigación científica. En química, se estudia por sus propiedades y reacciones químicas únicas. En biología y medicina, this compound se investiga ampliamente por sus efectos terapéuticos sobre la hipertensión y la enfermedad de las arterias coronarias. También se utiliza en estudios relacionados con sus propiedades antioxidantes y su capacidad para mejorar la producción de óxido nítrico, un vasodilatador crucial .
Análisis Bioquímico
Biochemical Properties
Amlodipine has antioxidant properties and an ability to enhance the production of nitric oxide (NO), an important vasodilator that decreases blood pressure . It interacts with calcium channels in the small arterioles, leading to arterial dilation .
Cellular Effects
Amlodipine exerts its action through inhibition of calcium influx into vascular smooth muscle cells and myocardial cells, which results in decreased peripheral vascular resistance . It also has a strong affinity for cell membranes, modulating calcium influx by inhibiting selected membrane calcium channels .
Molecular Mechanism
Amlodipine is a calcium channel blocker that inhibits the trans-membrane influx of calcium ions into vascular smooth muscle and cardiac muscle, leading to peripheral arterial vasodilation . This mechanism of action directly affects vascular smooth muscle to reduce peripheral vascular resistance, causing a decrease in blood pressure .
Temporal Effects in Laboratory Settings
Amlodipine has a high bioavailability, ranging from 60% to 80%, and undergoes hepatic metabolism. It is cleared only slowly by metabolism in the liver and has a long elimination half-life of 40 to 50 hours . This slow rate of elimination makes it suitable for single daily dosing .
Dosage Effects in Animal Models
In veterinary medicine, amlodipine is administered for treating high blood pressure, most commonly in cats. The effective dosage range in dogs is 0.1–0.2 mg/kg, orally, every 12 hours, or 0.2–0.4 mg/kg, orally, every 24 hours. The effective dosage range in cats is 0.625–1.25 mg/cat, orally, every 12–24 hours .
Metabolic Pathways
Amlodipine is extensively metabolised in the liver, but there is no significant presystemic or first-pass metabolism . It is slowly cleared with a terminal elimination half-life of 40 to 50 hours . The volume of distribution is large (21 L/kg) and there is a high degree of protein binding (98%) .
Transport and Distribution
Amlodipine undergoes gradual absorption from the gastrointestinal tract and is widely distributed throughout body tissues . It is cleared only slowly by metabolism in the liver and so has a long elimination half-life, which makes it suitable for single daily dosing .
Subcellular Localization
Amlodipine primarily affects the calcium channels in the small arterioles, leading to arterial dilation . It has been found that amlodipine can interfere with the specific locale of the endothelial nitric oxide synthase (eNOS) enzyme in cholesterol-rich plasmalemmal microdomains (e.g., caveolae and rafts), thereby modulating NO production in endothelial cells .
Métodos De Preparación
Rutas de síntesis y condiciones de reacción: Amlodipino se puede sintetizar mediante un proceso de varios pasos que implica la síntesis de dihidropiridina de Hantzsch. Los pasos clave incluyen la condensación de un aldehído, un éster β-ceto y una amina. Las condiciones de reacción suelen implicar calentar los reactivos en presencia de un disolvente adecuado .
Métodos de producción industrial: La producción industrial de this compound a menudo implica granulación húmeda y tableteado. El proceso incluye mezclar this compound besilato con excipientes como la celulosa microcristalina y la crospovidona, seguido de granulación, secado y tableteado .
Análisis De Reacciones Químicas
Tipos de reacciones: Amlodipino experimenta diversas reacciones químicas, incluida la oxidación, la reducción y la sustitución. Estas reacciones son esenciales para su metabolismo y degradación .
Reactivos y condiciones comunes: Los reactivos comunes utilizados en las reacciones que involucran this compound incluyen peróxido de hidrógeno para la oxidación y agentes reductores para las reacciones de reducción. Las condiciones a menudo implican temperaturas y niveles de pH controlados para garantizar los resultados deseados de la reacción .
Principales productos formados: Los principales productos formados a partir de las reacciones de this compound incluyen varios metabolitos de pirimidina inactivos. Estos metabolitos suelen excretarse del cuerpo a través de la orina .
Comparación Con Compuestos Similares
Amlodipino a menudo se compara con otros bloqueadores de los canales de calcio como nifedipino, diltiazem y verapamilo. A diferencia de estos compuestos, this compound tiene una duración de acción más larga y un inicio más lento, lo que lo hace adecuado para la dosificación una vez al día. Además, this compound tiene una cadena lateral única que mejora su selectividad para los vasos sanguíneos periféricos, lo que reduce el riesgo de depresión miocárdica y anormalidades de la conducción cardíaca .
Lista de compuestos similares:- Nifedipino
- Diltiazem
- Verapamilo
- Lisinopril (aunque no es un bloqueador de los canales de calcio, a menudo se compara por sus efectos antihipertensivos)
- Losartán (un bloqueador del receptor de la angiotensina, también se compara por sus efectos antihipertensivos)
Propiedades
IUPAC Name |
3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H25ClN2O5/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h5-8,17,23H,4,9-11,22H2,1-3H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HTIQEAQVCYTUBX-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC(=O)C1=C(NC(=C(C1C2=CC=CC=C2Cl)C(=O)OC)C)COCCN | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H25ClN2O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
88150-47-4 (maleate (1:1)) | |
| Record name | Amlodipine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0088150429 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID7022596 | |
| Record name | Amlodipine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022596 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
408.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Amlodipine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005018 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
slightly soluble in water | |
| Record name | Amlodipine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00381 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
**Mechanism of action on blood pressure** Amlodipine is considered a peripheral arterial vasodilator that exerts its action directly on vascular smooth muscle to lead to a reduction in peripheral vascular resistance, causing a decrease in blood pressure. Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the influx of calcium ions into both vascular smooth muscle and cardiac muscle. Experimental studies imply that amlodipine binds to both _dihydropyridine_ and _nondihydropyridine_ binding sites, located on cell membranes. The contraction of cardiac muscle and vascular smooth muscle are dependent on the movement of extracellular calcium ions into these cells by specific ion channels. Amlodipine blocks calcium ion influx across cell membranes with selectivity. A stronger effect of amlodipine is exerted on vascular smooth muscle cells than on cardiac muscle cells. Direct actions of amlodipine on vascular smooth muscle result in reduced blood pressure. **Mechanism of action in angina** The exact mechanism by which amlodipine relieves the symptoms of angina have not been fully elucidated to this date, however, the mechanism of action is likely twofold: Amlodipine has a dilating effect on peripheral arterioles, reducing the total peripheral resistance (afterload) against which the cardiac muscle functions. Since the heart rate remains stable during amlodipine administration, the reduced work of the heart reduces both myocardial energy use and oxygen requirements. Dilatation of the main coronary arteries and coronary arterioles, both in healthy and ischemic areas, is another possible mechanism of amlodipine reduction of blood pressure. The dilatation causes an increase in myocardial oxygen delivery in patients experiencing coronary artery spasm (Prinzmetal's or variant angina) and reduces coronary vasoconstriction caused by smoking., Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected in vitro but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect., Recent studies have suggested that cytokines are capable of modifying cardiovascular function and that drugs used in the treatment of heart failure have various modulating properties on the production of cytokines. More recently, we have found that ouabain induces the production of cytokines. This study was performed to examine the effects of calcium channel blockers on the production of cytokines induced by a cardiac glycoside. Human peripheral blood mononuclear cells (PBMC) were obtained from healthy volunteers. PBMC were cultured in 0.1, 1, 10, and 30 umol/L amlodipine, diltiazem, and nifedipine in presence of 1 umol/L ouabain. After 24 hr of incubation, IL-1alpha, IL-1beta, IL-6, and TNF-alpha were measured in the culture supernatants by enzyme-linked immunosorbent assay. Ouabain induced the production of IL-1alpha, IL-1beta and IL-6, but not of TNF-alpha. Induction of IL-1beta was most prominent. The production of IL-1alpha, and IL-6 was inhibited by amlodipine in a concentration-dependent manner and was significantly decreased at a concentration of 10 umol/L. IL-1beta production was also inhibited by 30 umol/L amlodipine. In contrast, neither diltiazem nor nifedipine inhibited the production of these cytokines. The unique property of amlodipine to inhibit the production of IL-1alpha, IL-1beta and IL-6 may contribute to its beneficial effects in heart failure patients., Proliferation of vascular smooth muscle cells (VSMC) contributes to the progression of atherosclerotic plaques. Calcium channel blockers have been shown to reduce VSMC proliferation, but the underlying molecular mechanism remains unclear. p21(Waf1/Cip1) is a potent inhibitor of cell cycle progression. Here, /investigators/ demonstrate that amlodipine (10(-6) to 10(-8) M) activates de novo synthesis of p21(Waf1/Cip1) in vitro. /Investigators/ show that amlodipine-dependent activation of p21(Waf1/Cip1) involves the action of the glucocorticoid receptor (GR) and C/EBP-alpha. The underlying pathway apparently involves the action of mitogen-activated protein kinase or protein kinase C, but not of extracellular signal-related kinase or changes of intracellular calcium. Amlodipine-induced p21(Waf1/Cip1) promoter activity and expression were abrogated by C/EBP-alpha antisense oligonucleotide or by the GR antagonist RU486. Amlodipine-dependent inhibition of cell proliferation was partially reversed by RU486 at 10(-8) M (58%+/-29%), antisense oligonucleotides targeting C/EBP-alpha (91%+/-26%), or antisense mRNAs targeting p21(Waf1/Cip1) (96%+/-32%, n=6); scrambled antisense oligonucleotides or those directed against C/EBP-beta were ineffective. The data suggest that the anti-proliferative action of amlodipine is achieved by induction of the p21 (Waf1/Cip1) gene, which may explain beneficial covert effects of this widely used cardiovascular therapeutic drug beyond a more limited role as a vascular relaxant., Calcium channel blockers (CCBs) are widely used in the therapy of cardiovascular diseases. Recent studies have shown that several CCBs exerted distinct anti-inflammatory effect in myocardial dysfunction models. The purpose of the present study was to evaluate therapeutic effect and possible mechanism of action of amlodipine, one of the widely used CCBs, on rat cardiac dysfunction during sepsis induced by lipopolysaccharide (LPS). Pretreatment of the rats with amlodipine (10 or 30 mg/kg, i.v.) delayed the fall of mean arterial blood pressure caused by LPS. Amlodipine also significantly inhibited the elevation of plasma tumor necrosis factor alpha (TNF-alpha) and decreased levels of inducible nitric oxide synthase (iNOS) in response to LPS challenge. To investigate the mechanism of the action of amlodipine, neonatal rat cardiomyocytes were used as a model. Amlodipine concentration-dependently decreased the release of TNF-alpha and iNOS protein expression, and suppressed the degradation and phosphorylation of inhibitor of kappaB-alpha (IkappaB-alpha) in LPS-activated neonatal rat cardiomyocytes. Further studies revealed that amlodipine markedly activated phosphatidylinositiol 3-kinase (PI3K) and Akt, downstream of the PI3K signal cascade. Application of PI3K inhibitors, wortmannin and LY294002 attenuated the depression of TNF-alpha and iNOS expression by amlodipine in LPS-induced cardiomyocytes. These findings may explain some cardioprotective effects of amlodipine in LPS-mediated sepsis and suggest that the inhibition of TNF-alpha and iNOS expression by amlodipine is, at least in part, dependent on PI3K/Akt signaling pathway. | |
| Record name | Amlodipine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00381 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | AMLODIPINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7079 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
88150-42-9 | |
| Record name | Amlodipine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=88150-42-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Amlodipine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0088150429 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Amlodipine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00381 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Amlodipine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022596 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 3-ethyl 5-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.102.428 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | 3,5-Pyridinedicarboxylic acid, 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl ester | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.125.844 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | AMLODIPINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1J444QC288 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | AMLODIPINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7079 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Amlodipine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005018 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
199-201 | |
| Record name | Amlodipine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00381 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods I
Procedure details






Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details




Synthesis routes and methods IV
Procedure details





Synthesis routes and methods V
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does Amlodipine exert its antihypertensive effect?
A1: Amlodipine primarily targets L-type calcium channels in vascular smooth muscle cells. [] By binding to these channels, it inhibits the influx of calcium ions, leading to vasodilation and a subsequent reduction in blood pressure. [, , ]
Q2: Does Amlodipine affect calcium channels in the heart?
A2: While Amlodipine exhibits vascular selectivity, it can also inhibit calcium channels in the heart, though to a lesser extent. [, ] This effect contributes to its antianginal properties by reducing myocardial oxygen demand.
Q3: How does the action of Amlodipine differ from other dihydropyridines?
A3: Amlodipine distinguishes itself through its slow rate of association and dissociation with its binding site on the calcium channel, leading to a gradual onset of action and a prolonged duration of effect. [, ] This characteristic makes it suitable for once-daily administration.
Q4: What is the molecular formula and weight of Amlodipine?
A4: Regrettably, the provided research excerpts do not explicitly state the molecular formula and weight of Amlodipine. Please refer to publicly available chemical databases for this information.
Q5: Is there any spectroscopic data available for Amlodipine within these research papers?
A5: The provided research excerpts do not delve into the spectroscopic characterization of Amlodipine.
Q6: Is there information regarding Amlodipine's compatibility with various materials or its stability under different conditions?
A6: The provided research predominantly focuses on the pharmacological aspects of Amlodipine. Information regarding material compatibility and stability under diverse conditions is not discussed.
Q7: Do the provided research papers indicate any catalytic properties or applications of Amlodipine?
A7: The research excerpts provided center around Amlodipine's therapeutic applications as a calcium channel blocker and do not suggest any catalytic properties.
Q8: Are there any mentions of computational studies, such as simulations or QSAR models, related to Amlodipine?
A8: The provided research excerpts do not discuss computational chemistry studies or modeling efforts concerning Amlodipine.
Q9: How do structural modifications of Amlodipine affect its activity?
A9: While the research doesn't directly compare structural analogs of Amlodipine, it highlights that its strong lipophilicity and positive charge contribute to a high affinity for calcium channels. [] Modifications impacting these properties could potentially alter its potency and duration of action.
Q10: What is the pharmacokinetic profile of Amlodipine?
A10: Amlodipine exhibits high oral bioavailability and a long elimination half-life, allowing for once-daily dosing. [] Studies have shown that Amlodipine does not undergo chiral transformation in healthy individuals. []
Q11: How does the pharmacokinetic profile of Amlodipine contribute to its long duration of action?
A11: The slow rate of association and dissociation of Amlodipine from its binding site on the calcium channel contributes to its prolonged duration of action. [, ]
Q12: What are the effects of Amlodipine on endothelial function?
A12: Research suggests that Amlodipine can improve endothelial function through dual mechanisms: by increasing endothelial nitric oxide (NO) bioavailability and by attenuating the formation of reactive oxygen species. []
Q13: Does Amlodipine impact atherogenesis?
A13: Studies in animal models have shown that Amlodipine can reduce the development of atheromatous lesions. [, ] Clinical studies with Amlodipine and other long-acting calcium channel blockers like Nifedipine and Lacidipine suggest similar anti-atherosclerotic effects in humans. [, , ]
Q14: What are the known resistance mechanisms for Amlodipine?
A14: The provided research excerpts do not delve into specific resistance mechanisms for Amlodipine.
Q15: Are there specific drug delivery strategies for targeting Amlodipine to specific tissues?
A15: The provided research excerpts primarily focus on the systemic effects of Amlodipine and do not explore targeted drug delivery strategies.
Q16: What analytical methods are used to quantify Amlodipine in biological samples?
A16: One study employed liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) for the chiral separation and quantification of Amlodipine enantiomers in human plasma. [] Another study used HPLC-DAD method. []
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.
![N-[(1S)-1-(4-tert-butylphenyl)ethyl]-2-(6,7-difluorobenzimidazol-1-yl)acetamide](/img/structure/B1665934.png)
![3-[5-(azetidine-1-carbonyl)pyrazin-2-yl]oxy-5-[(2S)-1-methoxypropan-2-yl]oxy-N-(5-methylpyrazin-2-yl)benzamide](/img/structure/B1665935.png)