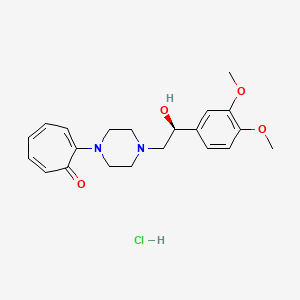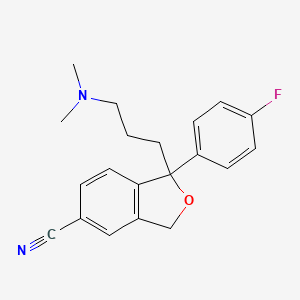
Citalopram
Descripción general
Descripción
Citalopram es un inhibidor selectivo de la recaptación de serotonina (ISRS) ampliamente utilizado como antidepresivo. Se prescribe principalmente para el tratamiento del trastorno depresivo mayor, el trastorno obsesivo-compulsivo, el trastorno de pánico y la fobia social . This compound mejora la transmisión serotoninérgica al inhibir la recaptación de serotonina, un neurotransmisor, en el cerebro .
Mecanismo De Acción
Citalopram actúa inhibiendo selectivamente la recaptación de serotonina en las neuronas presinápticas, lo que aumenta los niveles de serotonina en la hendidura sináptica . Esta acción mejora la transmisión serotoninérgica y alivia los síntomas de depresión y ansiedad . This compound tiene efectos mínimos en los transportadores de dopamina y norepinefrina y muestra poca o ninguna afinidad por otros subtipos de receptores .
Aplicaciones Científicas De Investigación
Citalopram tiene una amplia gama de aplicaciones de investigación científica:
Análisis Bioquímico
Biochemical Properties
Citalopram enhances serotonergic transmission through the inhibition of serotonin reuptake . Among all the SSRIs, this compound is the most selective toward serotonin reuptake inhibition . Specifically, it has a very minimal effect on dopamine and norepinephrine transportation and virtually no affinity for muscarinic, histaminergic, or GABAergic receptors .
Cellular Effects
This compound has been shown to have various effects on cells. For instance, it can cause side effects such as drowsiness, somnolence, hyponatremia, dizziness, and tachycardia . It can also treat depression, and panic disorders in adults .
Molecular Mechanism
The mechanism of action of this compound is presumed to be related to potentiation of serotonergic activity in the central nervous system (CNS) resulting from its inhibition of CNS neuronal reuptake of serotonin (5-HT), potentially through the inhibition of the serotonin transporter (solute carrier family 6 member 4, SLC6A4) .
Temporal Effects in Laboratory Settings
The therapeutic effects of all antidepressants, including this compound, develop over several weeks . This could be possibly as a result of adaptive changes in receptors . Adverse effects associated with this compound therapy include drowsiness, somnolence, hyponatremia, dizziness, and tachycardia .
Dosage Effects in Animal Models
In several animal models, this compound reduces serotonin turnover, presumably secondary to the increased intrasynaptic serotonin levels resulting from reuptake inhibition . This compound produced a mixed anxiogenic-/anxiolytic-like response in rats tested in the two-compartment black and white box .
Metabolic Pathways
This compound is metabolized mainly in the liver via N-demethylation to its main metabolite, demethylthis compound by CYP2C19 and CYP3A4 . Other metabolites include didemethylthis compound via CYP2D6 metabolism, this compound N-oxide and propionic acid derivative via monoamine oxidase enzymes A and B and aldehyde oxidase .
Transport and Distribution
This compound is extensively hepatic, via CYP3A4 and 2C19 (major pathways), and 2D6 (minor pathway); metabolized to demethylthis compound (DCT), didemethylthis compound (DDCT), this compound-N-oxide, and a deaminated propionic acid derivative, which are at least eight times less potent than this compound .
Subcellular Localization
Given its mechanism of action, it is likely that this compound is localized in the synaptic cleft where it inhibits the reuptake of serotonin, thereby increasing the concentration of serotonin in this region .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción
La síntesis de citalopram implica varios pasos clave:
Reducción de derivado de benzofenona: El proceso comienza con la reducción de una sal de magnesio no aislada de un derivado de benzofenona utilizando borohidruro de sodio en presencia de un solvente prótico.
Formación de compuesto intermedio: El compuesto reducido se hace reaccionar entonces con un catalizador ácido en un solvente no polar para obtener un compuesto intermedio.
Reacción con cianuro de cobre(I): El intermedio se hace reaccionar con cianuro de cobre(I) en un medio de solvente polar, seguido de recristalización utilizando solventes polares y/o alcohólicos para obtener el compuesto ciano.
Conversión final: El compuesto ciano se convierte entonces en this compound mediante métodos convencionales.
Métodos de producción industrial
La producción industrial de this compound sigue rutas sintéticas similares pero se optimiza para la fabricación a gran escala. El proceso implica un control estricto de las condiciones de reacción para asegurar un alto rendimiento y pureza del producto final .
Análisis De Reacciones Químicas
Tipos de reacciones
Citalopram sufre varias reacciones químicas, incluyendo:
Degradación térmica: This compound se descompone en un solo paso después de fundir a 189.3 °C, liberando bromuro de hidrógeno, dimetilamina y fluorobenceno.
Oxidación y reducción: Estas reacciones son menos comunes pero pueden ocurrir en condiciones específicas.
Reactivos y condiciones comunes
Borohidruro de sodio: Utilizado en el paso de reducción de la síntesis.
Cianuro de cobre(I): Utilizado en la formación del compuesto ciano.
Catalizadores ácidos: Empleados en el paso de formación intermedia.
Principales productos formados
Bromuro de hidrógeno: Liberado durante la degradación térmica.
Dimetilamina y fluorobenceno: Subproductos de la degradación térmica.
Comparación Con Compuestos Similares
Citalopram se compara con otros inhibidores selectivos de la recaptación de serotonina (ISRS) como:
Esthis compound: Más eficaz que el this compound para lograr una respuesta aguda y remisión en el trastorno depresivo mayor.
Fluoxetina: Eficacia similar pero perfiles de efectos secundarios diferentes.
Paroxetina: This compound es más eficaz y tiene un mejor perfil de efectos secundarios.
La singularidad de this compound radica en su alta selectividad para la inhibición de la recaptación de serotonina y su mínima interacción con otros sistemas de neurotransmisores .
Propiedades
IUPAC Name |
1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3H-2-benzofuran-5-carbonitrile | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WSEQXVZVJXJVFP-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCCC1(C2=C(CO1)C=C(C=C2)C#N)C3=CC=C(C=C3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H21FN2O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8022826 | |
| Record name | Citalopram | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8022826 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
324.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Citalopram | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005038 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
347-358, BP: 175-181 °C at 0.03 mm Hg /Citalopram/ | |
| Record name | Citalopram | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00215 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Citalopram | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7042 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
Sparingly soluble, log Kow = 1.39 at 22 °C.Solubility in water = 15,460 mg/L at 22 °C /Citalopram hydrobromide/, ... Sparingly soluble in water and soluble in ethanol. | |
| Record name | Citalopram | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00215 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Citalopram | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7042 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
The mechanism of action of citalopram results from its inhibition of CNS neuronal reuptake of serotonin (5-HT). The molecular target for citalopram is the serotonin transporter (solute carrier family 6 member 4, _SLC6A4_), inhibiting its serotonin reuptake in the synaptic cleft. Citalopram binds with significantly less affinity to histamine, acetylcholine, and norepinephrine receptors than tricyclic antidepressant drugs. This drug has no or neglible affinity for _5-HT1A_, _5-HT2A_, _dopamine D_1 and _D2_, _α1-_, _α2_-, and_ β adrenergic_, _histamine H1_, _gamma-aminobutyric acid_ (GABA), _muscarinic_, _cholinergic_, and _benzodiazepine_ receptors. Antagonism of _muscarinic_, _histaminergic_, and _adrenergic receptors_ is thought to be associated with several anticholinergic, sedative, and cardiovascular effects of other psychotropic drugs., /In/ whole-cell patch clamp recording of heterologous HERG-mediated currents in transfected mammalian cells ... citalopram blocks HERG with an IC(50) of 3.97 uM. This is slightly less potent than fluoxetine in /the same/ system (IC(50) of 1.50 uM). In isolated guinea pig ventricular cardiomyocytes, citalopram inhibited L-type calcium current (I(Ca,L)). The voltage dependence of I(Ca,L) inactivation in the presence of 100 uM citalopram was shifted significantly leftward. As a result, the I(Ca,L) 'window' in citalopram was found to be smaller & leftward-shifted compared to control. /These/ effects ... may help to explain citalopram's good cardiac safety profile, given its propensity to block HERG at excessive dosages. /Salt not specified/, The study was aimed to investigate the effects of the minimal effective doses of acute citalopram (5 mg/kg), (+/-)-8-hydroxydipropylaminotetralin HBr (8-OH-DPAT; 0.1 mg/kg), & their combined treatment on the rat open field & forced swimming behaviour & post-mortem monoamine content. The animals were prospectively divided into the vehicle- and para-chlorophenylalanine (p-CPA)-pretreated (350 mg/kg) groups. Acute citalopram (5 mg/kg), 8-OH-DPAT (0.1 mg/kg), or their combined treatment had no major effect on the rat open field & forced swimming behaviour. The post-mortem catecholamine content in four brain regions studied was unchanged in all treatment groups. The combined 8-OH-DPAT (0.1 mg/kg) & citalopram (5 mg/kg) treatment partially reversed the p-CPA-induced decr of serotonin (5-HT) and 5-hydroxy-indolacetic acid (5-HIAA) content. The present experiments demonstrate that the 5-HT1A receptors mediate some of the selective serotonin reuptake inhibitor-induced biochemical phenomena. | |
| Record name | Citalopram | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00215 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Citalopram | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7042 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Fine white to off-white powder | |
CAS No. |
59729-33-8 | |
| Record name | Citalopram | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=59729-33-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Citalopram [USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0059729338 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Citalopram | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00215 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Citalopram | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8022826 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Citalopram | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.056.247 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CITALOPRAM | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0DHU5B8D6V | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Citalopram | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7042 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Citalopram | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005038 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
182-188, Crystals from isopropanol. MP: 182-183. Freely soluble in water, ethanol, chloroform /Citolapram hydrobromide/, 178 °C | |
| Record name | Citalopram | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00215 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Citalopram | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7042 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Citalopram | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005038 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details







Synthesis routes and methods IV
Procedure details










Synthesis routes and methods V
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Citalopram?
A1: this compound primarily acts by inhibiting the serotonin transporter (SERT), a protein responsible for the reuptake of serotonin from the synaptic cleft back into the presynaptic neuron. This inhibition effectively increases the concentration of serotonin in the synapse, enhancing serotonergic neurotransmission. [, , ]
Q2: Does this compound interact with other molecular targets besides SERT?
A2: While this compound exhibits high selectivity for SERT, research indicates it can also interact with other molecular targets, including certain potassium channels. Notably, it inhibits TREK-1, TREK-2, and TRESK potassium channels, which are involved in regulating neuronal excitability. []
Q3: How does this compound's S-enantiomer, Esthis compound, differ in its interaction with SERT?
A3: Esthis compound demonstrates a higher affinity for SERT compared to the racemic this compound mixture. This difference in binding affinity contributes to its potentially faster onset of action and greater efficacy. [, , ]
Q4: What is the role of the R-enantiomer of this compound?
A4: The R-enantiomer of this compound, while not directly contributing to the therapeutic effect, has been shown to potentially inhibit the binding of the S-enantiomer to SERT, thereby potentially reducing its efficacy. []
Q5: How does chronic this compound treatment affect the adrenal gland's response to adrenocorticotropic hormone (ACTH)?
A5: Research suggests that chronic this compound treatment does not appear to sensitize the adrenal gland to ACTH in rats. While this compound-treated rats can elicit a corticosterone response to stress, this response is not accompanied by increased ACTH levels, suggesting alternative mechanisms may be involved. []
Q6: Has this compound been investigated for potential anti-cancer effects?
A6: Research indicates that this compound exhibits anti-proliferative activity against certain cancer cell lines, including the liver hepatocellular carcinoma cell line HepG2. This effect has been linked to the induction of apoptosis through mechanisms involving cytochrome C release and reactive oxygen species (ROS)-dependent activation of nuclear factor-κB (NF-κB). []
Q7: How do structural modifications of the this compound molecule influence its activity and selectivity?
A7: While the provided research papers do not extensively cover specific structural modifications and their impact, it is generally understood that even subtle alterations to the this compound molecule can significantly affect its binding affinity for SERT and other targets, potentially altering its pharmacological profile. This emphasizes the importance of SAR studies in drug development to optimize efficacy and minimize off-target effects. [General knowledge based on the field of medicinal chemistry]
Q8: How is this compound metabolized in the body?
A9: this compound is primarily metabolized in the liver by cytochrome P450 (CYP) enzymes, mainly CYP2C19, with CYP3A4 and CYP2D6 playing minor roles. The primary metabolite, desmethylthis compound, also exhibits pharmacological activity. [, , , ]
Q9: How does CYP2C19 genotype influence this compound metabolism and potential for adverse effects?
A10: Individuals classified as "slow metabolizers" due to genetic variations in the CYP2C19 gene may experience higher plasma concentrations of this compound compared to "normal metabolizers" receiving the same dose. This can lead to an increased risk of side effects and requires careful dose adjustments. [, , , ]
Q10: What in vitro models have been used to study the effects of this compound on neural cells?
A12: Rat PC12 cells have been utilized as an in vitro model to investigate the neuroprotective potential of this compound. Studies demonstrated that this compound exhibited anti-apoptotic effects on serum-deprived PC12 cells, potentially mediated by the upregulation of brain-derived neurotrophic factor (BDNF). []
Q11: What animal models have been used to investigate the effects of this compound on depression-related behaviors?
A13: The rat chronic mild stress (CMS) model, a well-established animal model of depression, has been employed to evaluate the antidepressant-like effects of this compound. Results showed that this compound effectively reversed CMS-induced behavioral deficits, further supporting its therapeutic potential. []
Q12: How does the time to response for this compound compare to other antidepressants in the rat CMS model?
A14: In the rat CMS model, this compound exhibited a faster onset of action compared to the tricyclic antidepressant imipramine and the SSRI fluoxetine, indicating potential advantages in terms of treatment response time. []
Q13: What cardiac effects have been associated with this compound, and what precautions are recommended?
A15: this compound, particularly at higher doses or in individuals with certain risk factors, has been associated with QTc interval prolongation on electrocardiograms (ECGs), a risk factor for potentially fatal ventricular arrhythmias like torsade de pointes. Regulatory bodies recommend dose adjustments and careful monitoring in patients with a history of cardiac conditions, electrolyte disturbances, or those taking medications known to prolong the QTc interval. [, , , ]
Q14: Is there a reliable ECG marker to predict the risk of ventricular arrhythmias in this compound intoxication?
A16: Research suggests that the QRS/QTc ratio, a novel ECG marker, shows promise in predicting ventricular arrhythmia risk in this compound intoxication. Patients experiencing ventricular arrhythmias exhibited significantly lower QRS/QTc ratios compared to those without arrhythmias. []
Q15: How does Esthis compound compare to other antidepressants in terms of efficacy and tolerability?
A17: Meta-analyses of clinical trials suggest that Esthis compound demonstrates comparable or superior efficacy to other SSRIs, such as this compound, Fluoxetine, Paroxetine, and Sertraline, in treating major depressive disorder. It may also have a more favorable tolerability profile compared to some other antidepressants. [, , , , ]
Q16: Are there non-pharmacological alternatives or adjunctive therapies for depression that can be considered?
A16: Yes, various non-pharmacological interventions, such as cognitive behavioral therapy (CBT), interpersonal therapy, and other forms of psychotherapy, have proven effective in treating depression. These therapies can be used alone or in conjunction with medication, depending on the individual patient's needs and preferences. [General knowledge based on the field of psychiatry]
Q17: What analytical methods are commonly employed for the quantification of this compound and its metabolites in biological samples?
A19: High-performance liquid chromatography (HPLC) coupled with various detection methods, such as ultraviolet (UV) detection or mass spectrometry (MS), is widely used for the accurate and sensitive quantification of this compound and its metabolites in plasma and other biological matrices. [, , ]
Q18: What sample preparation techniques are often used prior to HPLC analysis of this compound in biological samples?
A20: Solid-phase extraction (SPE) is a common sample preparation technique used to extract and purify this compound from biological matrices, such as plasma, prior to HPLC analysis. This technique helps to remove interfering compounds and concentrate the analyte of interest, improving the sensitivity and reliability of the analysis. [, ]
Q19: Have thin-layer chromatography (TLC) methods been explored for the analysis of this compound?
A21: Yes, TLC coupled with densitometric analysis has been investigated as a potential method for the simultaneous analysis of this compound and other centrally acting serotonin reuptake inhibitors. []
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.