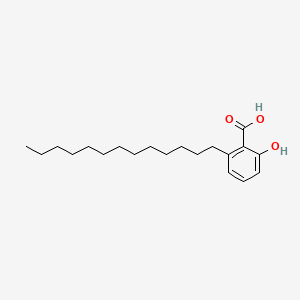
Glipizida
Descripción general
Descripción
La glipizida es un agente hipoglucémico oral que pertenece a la clase de fármacos de sulfonilurea de segunda generación. Se utiliza principalmente para controlar los niveles de azúcar en sangre en pacientes con diabetes mellitus tipo 2. La this compound funciona estimulando el páncreas para liberar insulina y aumentando la sensibilidad de los tejidos a la insulina . Se introdujo por primera vez en 1984 y está disponible bajo varios nombres comerciales, incluido Glucotrol .
Aplicaciones Científicas De Investigación
La glipizida tiene una amplia gama de aplicaciones de investigación científica, particularmente en los campos de la química, la biología, la medicina y la industria. En medicina, se utiliza para controlar los niveles de azúcar en sangre en pacientes con diabetes mellitus tipo 2 . En química, la this compound se estudia por su comportamiento térmico y sus formas polimórficas utilizando técnicas como la calorimetría de barrido diferencial (DSC), el análisis termogravimétrico (TGA), la difracción de rayos X en polvo (XRPD) y la espectroscopia infrarroja de transformada de Fourier (FT-IR) . Estos estudios ayudan a comprender el comportamiento de los sólidos farmacéuticos y garantizar la reproducibilidad de los lotes industriales .
Mecanismo De Acción
La glipizida ejerce sus efectos sensibilizando las células beta de los islotes de Langerhans pancreáticos a la respuesta a la insulina. Bloquea parcialmente los canales de potasio entre las células beta, lo que lleva a la despolarización celular y la apertura de los canales de calcio dependientes de voltaje. La afluencia de calcio resultante fomenta la liberación de insulina de las células beta . Este mecanismo ayuda a reducir los niveles de glucosa en sangre al promover la secreción de insulina y aumentar la sensibilidad de los tejidos a la insulina .
Análisis Bioquímico
Biochemical Properties
Glipizide functions as an insulin secretagogue, stimulating the release of insulin from the pancreatic beta cells, thereby increasing the plasma concentrations of insulin . This action is dependent on the functional beta cells in the pancreatic islets . Glipizide binds to the sulfonylurea receptor expressed on the pancreatic beta-cell plasma membrane, leading to the closure of the ATP-sensitive potassium channel and reduced potassium conductance .
Cellular Effects
Glipizide has a significant impact on various types of cells and cellular processes. It enhances the glucose uptake into the skeletal muscles and potentiates the action of insulin in the liver . Other effects include inhibited lipolysis in the liver and adipose tissue, inhibited hepatic glucose output, and increased uptake and oxidation of glucose .
Molecular Mechanism
The molecular mechanism of Glipizide involves partially blocking potassium channels among beta cells of pancreatic islets of Langerhans . By blocking potassium channels, the cell depolarizes, which results in the opening of voltage-gated calcium channels. The resulting calcium influx encourages insulin release from beta cells .
Temporal Effects in Laboratory Settings
Pharmacodynamic analysis indicates a significant relationship between plasma glipizide concentration and reduction in Fasting Plasma Glucose (FPG) and HbA1c over a dose range of 5–60 mg, with maximal efficacy achieved at a dose of 20 mg for FPG and at 5 mg for HbA1c .
Metabolic Pathways
Glipizide is metabolized primarily by hepatic transformation into several inactive metabolites by aromatic hydroxylation . A minor metabolite, an acetylamino-ethyl-benzene derivative, is reported to have some hypoglycemic activity .
Transport and Distribution
Glipizide is extensively bound to plasma albumin and has a relatively low volume of distribution . It is eliminated by metabolic biotransformation and excretion of the metabolites in the urine .
Subcellular Localization
The subcellular localization of Glipizide is primarily within the beta cells of the pancreatic islets of Langerhans . Here, it acts by partially blocking potassium channels, leading to cell depolarization and the subsequent release of insulin .
Métodos De Preparación
Rutas de síntesis y condiciones de reacción: La síntesis de glipizida implica varios pasos, comenzando desde la 4-[2-(5-metilpirazina-2-carboxamido)etil]bencenosulfonamida. Los pasos clave incluyen la cloración, seguida de la reacción con ciclohexilamina en presencia de un catalizador de hierro y monóxido de carbono . Este método es simple y práctico, y proporciona una nueva ruta de proceso para la síntesis de this compound .
Métodos de producción industrial: En entornos industriales, la this compound a menudo se prepara utilizando métodos de evaporación de disolventes para mejorar su solubilidad. Se preparan dispersiones sólidas de this compound utilizando polímeros como la polivinilpirrolidona (PVP) y el polietilenglicol (PEG). Las relaciones fármaco-polímero y las condiciones para la evaporación del disolvente se optimizan para lograr las tasas de solubilidad y disolución deseadas .
Análisis De Reacciones Químicas
Tipos de reacciones: La glipizida sufre varias reacciones químicas, incluida la oxidación, la reducción y la sustitución. Se sabe que el compuesto se descompone al calentarse, lo que lleva a la formación de 5-metil-N-[2-(4-sulfoamilfenil)etil]pirazina-2-carboxamida .
Reactivos y condiciones comunes: Los reactivos comunes utilizados en las reacciones que involucran this compound incluyen catalizadores de hierro, monóxido de carbono y ciclohexilamina . El proceso de descomposición implica la evolución de gases, incluidas la ciclohexanamina y el dióxido de carbono .
Productos principales: Los productos principales formados a partir de la descomposición de la this compound incluyen 5-metil-N-[2-(4-sulfoamilfenil)etil]pirazina-2-carboxamida .
Comparación Con Compuestos Similares
Compuestos similares: La glipizida a menudo se compara con otros fármacos de sulfonilurea como la glimepirida y la gliburida. También se compara con fármacos no sulfonilurea como la metformina y los miméticos de incretina como la semaglutida .
Singularidad: En comparación con otras sulfonilureas, la this compound tiene una rápida absorción y un inicio de acción rápido con la vida media y la duración de acción más cortas. Esto reduce el riesgo de hipoglucemia de larga duración, que a menudo se observa con otros agentes hipoglucémicos . Además, la estructura química única de la this compound, que incluye una cadena lateral no polar, aumenta su potencia hipoglucémica .
Propiedades
IUPAC Name |
N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-5-methylpyrazine-2-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H27N5O4S/c1-15-13-24-19(14-23-15)20(27)22-12-11-16-7-9-18(10-8-16)31(29,30)26-21(28)25-17-5-3-2-4-6-17/h7-10,13-14,17H,2-6,11-12H2,1H3,(H,22,27)(H2,25,26,28) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZJJXGWJIGJFDTL-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=CN=C(C=N1)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H27N5O4S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0040676 | |
| Record name | Glipizide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0040676 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
445.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Glipizide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015200 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>66.8 [ug/mL] (The mean of the results at pH 7.4), 1.64e-02 g/L | |
| Record name | SID855947 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Glipizide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01067 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Glipizide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015200 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder with increasing prevalence worldwide. Characterized by higher-than-normal levels of blood glucose, T2DM is a complex disorder that arises from the interaction between genetic, environmental and behavioral risk factors. Insulin is a peptide hormone that plays a critical role in regulating blood glucose levels. In response to high blood glucose levels, insulin promotes the uptake of glucose into the liver, muscle cells, and fat cells for storage. Although there are multiple events occurring that lead to the pathophysiology of T2DM, the disorder mainly involves insulin insensitivity as a result of insulin resistance, declining insulin production, and eventual failure of beta cells of pancreatic islets that normally produce insulin. Early management with lifestyle intervention, such as controlled diet and exercise, is critical in reducing the risk of long-term secondary complications, such as cardiovascular mortality. Glipizide, like other sulfonylurea drugs, is an insulin secretagogue, which works by stimulating the insulin release from the pancreatic beta cells thereby increasing the plasma concentrations of insulin. Thus, the main therapeutic action of the drug depends on the functional beta cells in the pancreatic islets. Sulfonylureas bind to the sulfonylurea receptor expressed on the pancreatic beta-cell plasma membrane, leading to the closure of the ATP-sensitive potassium channel and reduced potassium conductance. This results in depolarization of the pancreatic beta cell and opening of the voltage-sensitive calcium channels, promoting calcium ion influx. Increased intracellular concentrations of calcium ions in beta cells stimulates the secretion, or exocytosis, of insulin granules from the cells. Apart from this main mechanism of action, the blood-glucose-lowering effect of glipizide involves increased peripheral glucose utilization via stimulating hepatic gluconeogenesis and by increasing the number and sensitivity of insulin receptors. | |
| Record name | Glipizide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01067 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
29094-61-9 | |
| Record name | Glipizide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=29094-61-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Glipizide [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0029094619 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Glipizide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01067 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | glipizide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759120 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Glipizide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0040676 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Glipizide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.044.919 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | GLIPIZIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/X7WDT95N5C | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Glipizide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015200 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
200-203, 208 - 209 °C | |
| Record name | Glipizide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01067 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Glipizide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015200 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Glipizide?
A1: Glipizide exerts its hypoglycemic effect by binding to and blocking ATP-sensitive potassium (KATP) channels on the surface of pancreatic β-cells. [] This blockage depolarizes the β-cell membrane, leading to the opening of voltage-gated calcium channels. The subsequent influx of calcium ions triggers the exocytosis of insulin-containing granules, thereby increasing insulin secretion. [, ]
Q2: Does Glipizide only impact insulin secretion?
A2: While primarily recognized for enhancing insulin secretion, studies suggest that Glipizide might also influence insulin metabolism and peripheral glucose disposal. In obese type II diabetic patients, Glipizide treatment increased insulin metabolism, potentially within the liver. [] Additionally, it increased insulin-mediated peripheral glucose disposal in secondary failure type 2 diabetic patients. []
Q3: Can Glipizide affect glucose absorption?
A3: While initial studies suggested Glipizide might suppress xylose absorption in diet-controlled diabetics, further research revealed no such effect in insulin-dependent diabetics. [] This suggests that Glipizide's impact on postprandial glucose levels is primarily due to enhanced insulin secretion rather than altered absorption.
Q4: What is the molecular formula and weight of Glipizide?
A4: Glipizide (C21H27N5O4S) has a molecular weight of 445.55 g/mol. [, ]
Q5: Are there any spectroscopic data available for Glipizide?
A5: Yes, various spectroscopic techniques have been employed to characterize Glipizide. Fourier-transform infrared (FTIR) spectroscopy has been used to analyze Glipizide's stability and interactions with excipients in formulations like solid dispersions and bilayer tablets. [, , ] Additionally, differential scanning calorimetry (DSC) and X-ray diffraction (XRD) studies have revealed the amorphous nature of Glipizide in solid dispersions. []
Q6: What is the stability of Glipizide in various formulations?
A6: Glipizide stability has been investigated in various formulations. Studies demonstrate its stability in solid dispersions with low viscosity grade Hydroxypropyl methylcellulose (HPMCLV), even after storage. [] Bilayer tablet formulations with Hydroxypropyl methylcellulose (HPMC) and Ethyl Cellulose (EC) also exhibited stability according to ICH guidelines. []
Q7: Are there any known compatibility issues with Glipizide?
A7: While generally stable, certain drug interactions can impact Glipizide's effectiveness. For instance, co-administration with Nicorandil in diabetic mice hindered the glucose-lowering effects of Glipizide. [] Therefore, careful consideration is crucial when co-administering Glipizide with other medications.
Q8: How is Glipizide absorbed and metabolized?
A8: Glipizide is well-absorbed after oral administration. [] Food delays its absorption, unlike Glyburide. [] Glipizide undergoes extensive hepatic metabolism, and both metabolites and the unchanged drug are excreted in urine. []
Q9: What is the elimination half-life of Glipizide?
A9: Glipizide exhibits a short elimination half-life ranging from 2 to 7 hours. [] Its short half-life necessitates frequent dosing or the use of extended-release formulations to maintain therapeutic efficacy.
Q10: Are there any known drug interactions that affect Glipizide pharmacokinetics?
A11: Yes, several drugs can influence Glipizide pharmacokinetics. Clarithromycin significantly inhibits its metabolism in rabbits, leading to higher Glipizide exposure. [] Additionally, Esomeprazole, a proton pump inhibitor, can increase Glipizide concentration and AUC (area under the curve) by inhibiting Cytochrome P-450 enzymes responsible for its metabolism. []
Q11: What animal models have been used to investigate the effects of Glipizide?
A11: Various animal models have been employed, including:
- Diabetic-prone BB rats: Demonstrated Glipizide's ability to prevent diabetes and reduce islet inflammation. []
- Healthy and diabetic rabbits: Used to study the influence of drugs like Clarithromycin [] and Puerarin [] on Glipizide pharmacokinetics.
- Healthy cats: Investigated transdermal application of Glipizide. []
- Healthy and endotoxemic sheep: Studied the effects of Glipizide on cardiopulmonary hemodynamics and oxygen transport. []
- Alloxan-induced diabetic rats: Assessed the combined effect of Glipizide and alpha lipoic acid on diabetic parameters and hepatic function. []
Q12: How effective is Glipizide in controlling blood glucose levels compared to other treatments?
A12: Studies have compared Glipizide's efficacy to other treatments:
- Glyburide: In a Veterans Health Administration study, converting from Glyburide to Glipizide increased HbA1c levels, but reduced the incidence of hypoglycemia. []
Q13: How does Glipizide's low solubility affect its formulation?
A15: Glipizide's low solubility (BCS Class II drug) poses challenges for formulation and can limit its bioavailability. [, ] Various strategies have been explored to enhance its solubility and dissolution rate.
Q14: What formulation strategies have been investigated to improve Glipizide delivery?
A14: Several approaches have been explored to enhance Glipizide delivery:
- Solid Dispersions: Utilizing low viscosity grade HPMCLV improved Glipizide's dissolution rate. []
- Nano-complexes: Glipizide-Phospholipid nano-complexes demonstrated improved solubility and bioavailability in rats. []
- Microspheres: Ethyl Cellulose microspheres showed potential for sustained Glipizide release. []
- Transdermal Delivery: Studies explored transdermal patches incorporating Glipizide-cyclodextrin complexes and penetration enhancers, achieving sustained drug release and therapeutic effects in diabetic rats. []
Q15: Have any specific cyclodextrins shown promise in enhancing Glipizide solubility?
A17: Yes, complexation with cyclodextrins, especially Dimethyl-beta-cyclodextrin (DM-beta-CyD), significantly improved Glipizide release and permeation through rat skin in transdermal formulations. []
Q16: What is the rationale behind developing a transdermal delivery system for Glipizide?
A18: Transdermal delivery of Glipizide aims to address the limitations of oral therapy, such as fluctuating bioavailability, risk of severe hypoglycemia, and gastric disturbances. []
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![(1R,3R,6R,7S,8S,9R,10S,13S,16S,17R)-8-tert-butyl-6,9,17-trihydroxy-16-methyl-2,4,14,19-tetraoxahexacyclo[8.7.2.01,11.03,7.07,11.013,17]nonadecane-5,15,18-trione](/img/structure/B1671515.png)