Levotiroxina
Descripción general
Descripción
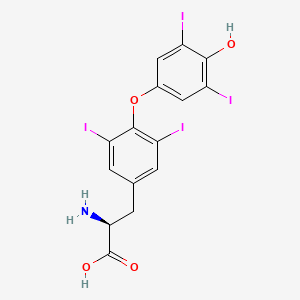
La levotiroxina, también conocida como L-tiroxina, es una forma sintética de la hormona tiroidea tiroxina (T4). Se utiliza principalmente para tratar la deficiencia de hormona tiroidea (hipotiroidismo), incluidas las formas graves como el coma mixedematoso. La this compound también se usa para tratar y prevenir ciertos tipos de tumores tiroideos . Está disponible en varias formas, incluyendo tabletas orales, inyecciones intravenosas y soluciones líquidas .
Aplicaciones Científicas De Investigación
La levotiroxina tiene una amplia gama de aplicaciones de investigación científica:
Química: Se utiliza como compuesto de referencia en química analítica para el desarrollo de nuevos métodos analíticos.
Biología: Se estudia su papel en la regulación del metabolismo y el crecimiento en varios organismos.
Medicina: Se usa ampliamente en el tratamiento del hipotiroidismo y como terapia supresora para los tumores tiroideos.
Industria: Se emplea en la industria farmacéutica para la producción de terapias de reemplazo de hormona tiroidea.
Mecanismo De Acción
La levotiroxina ejerce sus efectos reemplazando la hormona tiroidea que normalmente produce la glándula tiroides. Se convierte en triyodotironina (T3) en los tejidos periféricos, que luego se une a los receptores de la hormona tiroidea en el núcleo de las células. Esta unión activa la transcripción genética y la síntesis de proteínas, lo que lleva a un aumento del metabolismo, el crecimiento y el desarrollo . Los objetivos moleculares primarios son los receptores de la hormona tiroidea, y las vías involucradas incluyen la regulación de los procesos metabólicos y el gasto energético .
Análisis Bioquímico
Biochemical Properties
Levothyroxine plays a crucial role in various biochemical reactions. It interacts with numerous enzymes, proteins, and other biomolecules . The regulation of thyroid hormones within the hypothalamic-pituitary-thyroid axis is complex, consisting of multiple feedback and feed-forward loops .
Cellular Effects
Levothyroxine influences various types of cells and cellular processes. It impacts cell function, including effects on cell signaling pathways, gene expression, and cellular metabolism . Levothyroxine replacement therapy for people with hypothyroidism reverses many metabolic disturbances associated with hypothyroidism .
Molecular Mechanism
Levothyroxine exerts its effects at the molecular level through binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . Exogenous Levothyroxine is indistinguishable from endogenous T4 .
Temporal Effects in Laboratory Settings
The effects of Levothyroxine change over time in laboratory settings. Information on the product’s stability, degradation, and any long-term effects on cellular function observed in in vitro or in vivo studies is currently being researched .
Dosage Effects in Animal Models
The effects of Levothyroxine vary with different dosages in animal models. Studies are ongoing to determine any threshold effects observed in these studies, as well as any toxic or adverse effects at high doses .
Metabolic Pathways
Levothyroxine is involved in various metabolic pathways, interacting with enzymes or cofactors. It also affects metabolic flux or metabolite levels .
Transport and Distribution
Levothyroxine is transported and distributed within cells and tissues. It interacts with transporters or binding proteins, affecting its localization or accumulation .
Subcellular Localization
The subcellular localization of Levothyroxine and its effects on activity or function are areas of active research. This includes any targeting signals or post-translational modifications that direct it to specific compartments or organelles .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La levotiroxina se puede sintetizar a través de varios métodos. Un método común implica la yodación de la 3,5-diiodotironina. Este proceso incluye la desmetilación del ácido 2-amino-3-(3,5-diiodo-4-(4-metoxifenoxi)fenil)propanoico usando una mezcla de ácido acético y ácido yodhídrico para dar 3,5-diiodotironina, que luego se yoda para producir this compound .
Métodos de producción industrial: La producción industrial de this compound implica procesos de varios pasos para garantizar un alto rendimiento y pureza. Un método incluye el uso de grupos protectores en el éster etílico de (S)-N-acetil-3,5-diiodo-4-p-metoxifenoxifenilalanina, que se escinde mediante una mezcla de ácido yodhídrico y ácido bromhídrico para dar 3,5-diiodotironina. La yodación posterior con yodo produce this compound con un rendimiento de aproximadamente el 92% .
Análisis De Reacciones Químicas
Tipos de reacciones: La levotiroxina experimenta varias reacciones químicas, que incluyen:
Oxidación: La this compound se puede oxidar para formar diferentes derivados.
Reducción: Las reacciones de reducción pueden modificar los átomos de yodo en la molécula.
Sustitución: Las reacciones de sustitución pueden ocurrir en el grupo hidroxilo fenólico o en el grupo amino.
Reactivos y condiciones comunes:
Oxidación: Los agentes oxidantes comunes incluyen peróxido de hidrógeno y yodo.
Reducción: Se pueden usar agentes reductores como el borohidruro de sodio.
Sustitución: Los reactivos como los haluros de alquilo y los cloruros de acilo se utilizan comúnmente para las reacciones de sustitución.
Productos principales formados:
Oxidación: Derivados oxidados de this compound.
Reducción: Formas reducidas de this compound con menos átomos de yodo.
Sustitución: Derivados sustituidos con diferentes grupos funcionales.
Comparación Con Compuestos Similares
La levotiroxina a menudo se compara con otras hormonas tiroideas y análogos sintéticos:
Triyodotironina (T3): La this compound (T4) se convierte en triyodotironina (T3) en el cuerpo.
Liotironina: Una forma sintética de T3, utilizada para la aparición rápida de la acción en pacientes hipotiroideos.
Extracto de tiroides desecado: Contiene tanto T4 como T3 en una proporción que difiere de la secreción tiroidea humana.
La this compound es única en su estabilidad, larga vida media y capacidad de proporcionar niveles consistentes de hormona tiroidea con una dosis única diaria .
Propiedades
IUPAC Name |
(2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]propanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C15H11I4NO4/c16-8-4-7(5-9(17)13(8)21)24-14-10(18)1-6(2-11(14)19)3-12(20)15(22)23/h1-2,4-5,12,21H,3,20H2,(H,22,23)/t12-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XUIIKFGFIJCVMT-LBPRGKRZSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=C(C=C(C(=C1I)OC2=CC(=C(C(=C2)I)O)I)I)CC(C(=O)O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1=C(C=C(C(=C1I)OC2=CC(=C(C(=C2)I)O)I)I)C[C@@H](C(=O)O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C15H11I4NO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8023214 | |
| Record name | Levothyroxine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023214 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
776.87 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Thyroxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000248 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Slightly soluble in water, Insoluble in ethanol, benzene | |
| Record name | Levothyroxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00451 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LEVOTHYROXINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3108 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Levothyroxine is a synthetically prepared levo-isomer of the thyroid hormone thyroxine (T4, a tetra-iodinated tyrosine derivative) that acts as a replacement in deficiency syndromes such as hypothyroidism. T4 is the major hormone secreted from the thyroid gland and is chemically identical to the naturally secreted T4: it increases metabolic rate, decreases thyroid-stimulating hormone (TSH) production from the anterior lobe of the pituitary gland, and, in peripheral tissues, is converted to T3. Thyroxine is released from its precursor protein thyroglobulin through proteolysis and secreted into the blood where is it then peripherally deiodinated to form triiodothyronine (T3) which exerts a broad spectrum of stimulatory effects on cell metabolism. T4 and T3 have a relative potency of ~1:4. Thyroid hormone increases the metabolic rate of cells of all tissues in the body. In the fetus and newborn, thyroid hormone is important for the growth and development of all tissues including bones and the brain. In adults, thyroid hormone helps to maintain brain function, food metabolism, and body temperature, among other effects. The symptoms of thyroid deficiency relieved by levothyroxine include slow speech, lack of energy, weight gain, hair loss, dry thick skin and unusual sensitivity to cold. The thyroid hormones have been shown to exert both genomic and non-genomic effects. They exert their genomic effects by diffusing into the cell nucleus and binding to thyroid hormone receptors in DNA regions called thyroid hormone response elements (TREs) near genes. This complex of T4, T3, DNA, and other coregulatory proteins causes a conformational change and a resulting shift in transcriptional regulation of nearby genes, synthesis of messenger RNA, and cytoplasmic protein production. For example, in cardiac tissues T3 has been shown to regulate the genes for α- and β-myosin heavy chains, production of the sarcoplasmic reticulum proteins calcium-activated ATPase (Ca2+-ATPase) and phospholamban, β-adrenergic receptors, guanine-nucleotide regulatory proteins, and adenylyl cyclase types V and VI as well as several plasma-membrane ion transporters, such as Na+/K+–ATPase, Na+/Ca2+ exchanger, and voltage-gated potassium channels, including Kv1.5, Kv4.2, and Kv4.3. As a result, many cardiac functions including heart rate, cardiac output, and systemic vascular resistance are closely linked to thyroid status. The non-genomic actions of the thyroid hormones have been shown to occur through binding to a plasma membrane receptor integrin aVb3 at the Arg-Gly-Asp recognition site. From the cell-surface, T4 binding to integrin results in down-stream effects including activation of mitogen-activated protein kinase (MAPK; ERK1/2) and causes subsequent effects on cellular/nuclear events including angiogenesis and tumor cell proliferation. | |
| Record name | Levothyroxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00451 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Color/Form |
Crystals, Needles | |
CAS No. |
51-48-9 | |
| Record name | (-)-Thyroxine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=51-48-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Levothyroxine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000051489 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Levothyroxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00451 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | levothyroxine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757434 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Levothyroxine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023214 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | LEVOTHYROXINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/Q51BO43MG4 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | LEVOTHYROXINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3108 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thyroxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000248 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
Decomposes at 235-236 °C, 235.5 °C | |
| Record name | Levothyroxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00451 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LEVOTHYROXINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3108 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thyroxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000248 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.