Mebendazol
Descripción general
Descripción
El mebendazol es un compuesto antihelmíntico de amplio espectro utilizado para tratar diversas infestaciones de gusanos parásitos. Fue desarrollado por Janssen Pharmaceutica en Bélgica y entró en uso en 1971 . El this compound es efectivo contra una gama de infestaciones de nematodos, incluyendo ascárides, anquilostomas, tricocéfalos y oxiuros . Se absorbe mal en el torrente sanguíneo, lo que lo hace particularmente efectivo para tratar parásitos intestinales .
Mecanismo De Acción
El mebendazol funciona inhibiendo la polimerización de la tubulina en los parásitos, interrumpiendo la formación de microtúbulos e interfiriendo con la absorción de glucosa . Esto conduce en última instancia a la muerte del parásito. El compuesto se dirige a vías críticas implicadas en la proliferación celular, la apoptosis y la invasión/migración .
Aplicaciones Científicas De Investigación
El mebendazol se ha reutilizado para diversas aplicaciones de investigación científica más allá de su uso original como antihelmíntico. Ha mostrado promesa en el tratamiento de cánceres cerebrales, incluyendo el glioma, al inhibir la progresión maligna y aumentar la sensibilidad de las células gliomatosas a la quimioterapia o radioterapia convencionales . Además, se ha explorado el this compound por sus propiedades anticancerígenas en múltiples cánceres, incluyendo la leucemia mieloide aguda, el cáncer de mama y el cáncer gastrointestinal .
Análisis Bioquímico
Biochemical Properties
Mebendazole functions by interfering with the polymerization of tubulin, a protein essential for microtubule formation. This disruption leads to the loss of cytoplasmic microtubules, which are crucial for various cellular processes, including cell division and intracellular transport . Mebendazole interacts with beta-tubulin, inhibiting its polymerization and thereby affecting the stability of microtubules . Additionally, Mebendazole has been shown to inhibit glucose uptake in parasitic worms, leading to their death .
Cellular Effects
Mebendazole exerts significant effects on various cell types and cellular processes. In cancer cells, Mebendazole induces apoptosis by disrupting microtubule dynamics and causing cell cycle arrest at the G2/M phase . It also affects cell signaling pathways, including the hedgehog signaling pathway, which is involved in cell proliferation and survival . Mebendazole has been shown to decrease the expression of genes associated with cell proliferation and increase the expression of pro-apoptotic genes . Furthermore, Mebendazole influences cellular metabolism by inhibiting glucose uptake and disrupting mitochondrial function .
Molecular Mechanism
At the molecular level, Mebendazole binds to beta-tubulin, inhibiting its polymerization and disrupting microtubule formation . This inhibition leads to the destabilization of microtubules, which are essential for cell division and intracellular transport . Mebendazole also affects other molecular targets, including the Bcl-2 family of proteins, which regulate apoptosis . By inhibiting Bcl-2, Mebendazole promotes the release of cytochrome c from mitochondria, triggering the apoptotic cascade . Additionally, Mebendazole has been shown to inhibit angiogenesis by targeting vascular endothelial growth factor receptor 2 (VEGFR2) signaling .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Mebendazole have been observed to change over time. Mebendazole is relatively stable, but its efficacy can decrease due to degradation . Long-term studies have shown that Mebendazole can cause sustained inhibition of tumor growth and metastasis in in vivo models . The stability and degradation of Mebendazole can vary depending on the experimental conditions and the presence of other compounds .
Dosage Effects in Animal Models
The effects of Mebendazole vary with different dosages in animal models. At low doses, Mebendazole effectively inhibits parasitic worm infections without causing significant toxicity . At higher doses, Mebendazole can cause adverse effects, including bone marrow suppression and hepatotoxicity . In cancer models, higher doses of Mebendazole have been shown to inhibit tumor growth and metastasis more effectively . The therapeutic window for Mebendazole is relatively wide, but careful dose optimization is necessary to minimize toxicity .
Metabolic Pathways
Mebendazole is metabolized primarily in the liver by cytochrome P450 enzymes . The major metabolic pathways involve oxidation and reduction reactions, leading to the formation of various metabolites . These metabolites can have different pharmacological activities and contribute to the overall efficacy and toxicity of Mebendazole . The metabolic flux and levels of metabolites can vary depending on the dose and duration of treatment .
Transport and Distribution
Mebendazole is poorly absorbed from the gastrointestinal tract, resulting in low systemic bioavailability . Once absorbed, Mebendazole is extensively distributed in tissues, including the liver, lungs, and kidneys . It is transported in the bloodstream bound to plasma proteins, primarily albumin . Mebendazole can also penetrate the blood-brain barrier, making it effective against central nervous system infections and tumors .
Subcellular Localization
Mebendazole localizes primarily in the cytoplasm, where it interacts with microtubules . It does not have specific targeting signals or post-translational modifications that direct it to specific organelles . Its effects on microtubule dynamics can influence the distribution and function of other cellular components, including mitochondria and the endoplasmic reticulum . The disruption of microtubules by Mebendazole can lead to changes in the organization and function of these organelles .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción
La preparación del mebendazol implica una reacción de acilación seguida de una reacción de Friedel-Crafts. En la reacción de acilación, el triclorotolueno se calienta a 50-90 °C, y se añade gota a gota una solución acuosa de cloruro de zinc. Después de la reacción, se realiza una destilación a presión reducida para obtener cloruro de benzoílo . En la reacción de Friedel-Crafts, se mezclan y agitan carbendazim, un disolvente y cloruro de aluminio anhidro. A continuación, se añade gota a gota cloruro de benzoílo, seguido de agitación continua y reacción de conservación del calor. Se realiza una destilación a presión reducida para obtener this compound .
Métodos de producción industrial
La producción industrial del this compound suele seguir la misma ruta sintética que se ha descrito anteriormente. El proceso se optimiza para obtener un alto rendimiento y eficiencia, con especial atención a un flujo simple, condiciones suaves y una alta tasa de utilización de átomos .
Análisis De Reacciones Químicas
Tipos de reacciones
El mebendazol experimenta diversas reacciones químicas, incluyendo reacciones de oxidación, reducción y sustitución.
Reactivos y condiciones comunes
Oxidación: Se pueden utilizar oxidantes comunes como el peróxido de hidrógeno o el permanganato de potasio.
Reducción: Se pueden emplear agentes reductores como el catalizador de paladio sobre carbón en presencia de hidrógeno.
Sustitución: Las reacciones de sustitución suelen implicar el uso de agentes halogenantes o nucleófilos.
Productos principales
Los productos principales que se forman a partir de estas reacciones dependen de los reactivos y las condiciones específicas que se utilicen. Por ejemplo, la reducción de la 4-amino-3-nitrobenzofenona con catalizador de paladio sobre carbón produce 3,4-diaminobenzofenona .
Comparación Con Compuestos Similares
Compuestos similares
Albendazol: Otro antihelmíntico benzimidazol utilizado para tratar una variedad de infestaciones de gusanos parásitos.
Pirantel: Un antihelmíntico utilizado para tratar infecciones por oxiuros, anquilostomas y ascárides.
Singularidad
El mebendazol es único en su capacidad para penetrar la barrera hematoencefálica, lo que lo hace particularmente efectivo para tratar tumores cerebrales . Su actividad de amplio espectro y su toxicidad relativamente baja también lo distinguen de otros compuestos similares .
Propiedades
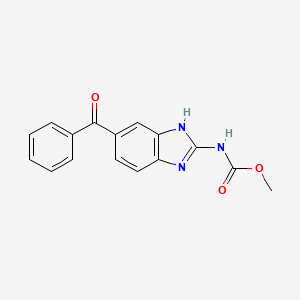
IUPAC Name |
methyl N-(6-benzoyl-1H-benzimidazol-2-yl)carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H13N3O3/c1-22-16(21)19-15-17-12-8-7-11(9-13(12)18-15)14(20)10-5-3-2-4-6-10/h2-9H,1H3,(H2,17,18,19,21) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OPXLLQIJSORQAM-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC(=O)NC1=NC2=C(N1)C=C(C=C2)C(=O)C3=CC=CC=C3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H13N3O3 | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID4040682 | |
| Record name | Mebendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4040682 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
295.29 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Mebendazole is a white to slightly yellow powder. Pleasant taste. Practically water insoluble. (NTP, 1992), Solid | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Practically insoluble (NTP, 1992), Soluble in formic acid. Practically insoluble in ethanol, ether, chloroform, In water, 7.13X10+1 mg/L at 25 °C, 3.87e-02 g/L | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Mebendazole causes degenerative alterations in the tegument and intestinal cells of the worm by binding to the colchicine-sensitive site of tubulin, thus inhibiting its polymerization or assembly into microtubules. The loss of the cytoplasmic microtubules leads to impaired uptake of glucose by the larval and adult stages of the susceptible parasites, and depletes their glycogen stores. Degenerative changes in the endoplasmic reticulum, the mitochondria of the germinal layer, and the subsequent release of lysosomes result in decreased production of adenosine triphosphate (ATP), which is the energy required for the survival of the helminth. Due to diminished energy production, the parasite is immobilized and eventually dies., Although the exact mechanism of anthelmintic activity of mebendazole has not been fully elucidated, the drug appears to cause selective and irreversible inhibition of the uptake of glucose and other low molecular weight nutrients in susceptible helminths; inhibition of glucose uptake appears to result in endogenous depletion of glycogen stores in the helminth. Mebendazole does not inhibit glucose uptake in mammals. Mebendazole appears to cause degenerative changes in the intestine of nematodes and in the absorptive cells of cestodes. The principal anthelmintic effect of the drug appears to be degeneration of cytoplasmic microtubules within these intestinal and absorptive cells. Microtubular deterioration results in inhibition of organelle movement and interferes with the absorptive and secretory function. As a result of excessive accumulation of intracellular transport secretory granules, hydrolytic and proteolytic enzymes are released and cause cellular autolysis. This irreversible damage leads to death of the parasite., Vermicidal; may also be ovicidal for ova or most helminths; mebendazole causes degeneration of parasite's cytoplasmic microtubules and thereby selectively and irreversibly blocks glucose uptake in susceptible adult intestine-dwelling helminths and their tissue-dwelling larvae; inhibition of glucose uptake apparently results in depletion of the parasite's glycogen stores; this, in turn, results in reduced formation of adenosine triphosphate (ATP) required for survival and reproduction of the helminth; corresponding energy levels are gradually reduced until death of the parasite ensues; mebendazole does not appear to affect serum glucose concentrations in humans, however., Benzimidazoles produce many biochemical changes in susceptible nematodes, eg, inhibition of mitochondrial fumarate reductase, reduced glucose transport, and uncoupling of oxidative phosphorylation ... /but/ the primary action ... /should be/ to inhibit microtubule polymerization by binding to beta-tubulin. The selective toxicity of these agents derives from the fact that specific, high-affinity binding to parasite beta-tubulin occurs at much lower concn than does binding to the mammalian protein ... Benzimidazole-resistant Haemonchus contortus display reduced high-affinity drug binding to beta-tubulin and alterations in beta-tubulin isotype gene expression that correlate with drug resistance ... Two identified mechanisms of drug resistance in nematodes involve both a progressive loss of "susceptible" beta-tubulin gene isotypes together with emergence of a "resistant" isotype with a conserved point mutation that encodes a tyrosine instead of phenylalanine at position 200 of beta-tubulin. While this mutation may not be required for benzimidazole resistance in all parasites, eg, Giardia lamblia, benzimidazole resistance in parasitic nematodes is unlikely to be overcome by novel benzimidazole analogs, because tyrosine also is present at position 200 of human beta-tubulin. /Benzimidazoles/ | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Off-white amorphous powder, Crystals from acetic acid and methanol | |
CAS No. |
31431-39-7 | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=31431-39-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Mebendazole [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0031431397 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | mebendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757838 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | mebendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=184849 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Mebendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4040682 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Mebendazole | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.046.017 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MEBENDAZOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/81G6I5V05I | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
551.3 °F (NTP, 1992), 288.5 °C | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.