Nateglinida
Descripción general
Descripción
Nateglinide is an oral antihyperglycemic agent used for the treatment of non-insulin-dependent diabetes mellitus (type 2 diabetes). It belongs to the meglitinide class of short-acting insulin secretagogues, which act by binding to beta cells of the pancreas to stimulate insulin release . Nateglinide was developed by Ajinomoto, a Japanese company, and is marketed under the trade name Starlix by the Swiss pharmaceutical company Novartis .
Mecanismo De Acción
Target of Action
Nateglinide is an oral antihyperglycemic agent used for the treatment of non-insulin-dependent diabetes mellitus (NIDDM). It belongs to the meglitinide class of short-acting insulin secretagogues, which act by binding to β cells of the pancreas to stimulate insulin release . The primary targets of Nateglinide are the β cells in the pancreas .
Mode of Action
Nateglinide induces an early insulin response to meals, decreasing postprandial blood glucose levels . It achieves this by closing ATP-dependent potassium channels in the membrane of the β cells . This depolarizes the β cells and causes voltage-gated calcium channels to open . The influx of calcium ions stimulates calcium-dependent exocytosis of insulin granules .
Biochemical Pathways
Nateglinide affects the insulin secretion pathway. By closing the ATP-dependent potassium channels in the β cells, it causes depolarization. This leads to the opening of voltage-gated calcium channels, and the resulting influx of calcium ions induces the exocytosis of insulin granules . This process is dependent on the presence of functioning β cells and glucose .
Pharmacokinetics
Nateglinide is extensively metabolized in the liver and excreted in urine (83%) and feces (10%) . The major metabolites possess less activity than the parent compound . One minor metabolite, the isoprene, has the same potency as its parent compound .
Result of Action
The primary result of Nateglinide’s action is a decrease in postprandial and fasting blood glucose levels . It also decreases glycosylated hemoglobin (HbA1c) levels, which are reflective of the last 8-10 weeks of glucose control . Nateglinide is more effective at lowering postprandial blood glucose than metformin, sulfonylureas, and thiazolidinediones .
Action Environment
The action of Nateglinide is dependent on the presence of glucose . Therefore, its efficacy can be influenced by the patient’s diet. It should only be taken with meals, and meal-time doses should be skipped with any skipped meal . This ensures that the drug’s action is synchronized with the body’s glucose levels, maximizing its efficacy and minimizing the risk of hypoglycemia .
Aplicaciones Científicas De Investigación
Nateglinide has several scientific research applications, including:
Análisis Bioquímico
Biochemical Properties
Nateglinide plays a crucial role in biochemical reactions by stimulating the secretion of insulin. It achieves this by binding to ATP-sensitive potassium channels on the pancreatic beta cells, leading to their closure . This action depolarizes the beta cells and opens voltage-gated calcium channels, resulting in an influx of calcium ions . The increased intracellular calcium concentration triggers the exocytosis of insulin-containing vesicles, thereby promoting insulin release . Nateglinide interacts primarily with the ATP-sensitive potassium channels and voltage-gated calcium channels in this process .
Cellular Effects
Nateglinide influences various cellular processes, particularly in pancreatic beta cells. By stimulating insulin release, it helps regulate blood glucose levels . This regulation impacts cell signaling pathways related to glucose metabolism and insulin signaling. Nateglinide’s action on ATP-sensitive potassium channels and subsequent calcium influx affects gene expression and cellular metabolism by promoting insulin secretion . Additionally, nateglinide has been shown to have minimal effects on insulin levels between meals and overnight, making it a targeted therapy for postprandial glucose control .
Molecular Mechanism
At the molecular level, nateglinide exerts its effects by binding to and inhibiting ATP-sensitive potassium channels on pancreatic beta cells . This inhibition leads to membrane depolarization and the opening of voltage-gated calcium channels . The resulting calcium influx triggers the fusion of insulin-containing vesicles with the cell membrane, facilitating insulin secretion . Nateglinide’s mechanism of action is glucose-dependent, meaning it enhances insulin release in the presence of glucose but has little effect when glucose levels are low .
Temporal Effects in Laboratory Settings
In laboratory settings, nateglinide exhibits rapid absorption and a short half-life of approximately 1.5 hours . Its effects on insulin secretion are quick and short-lived, aligning with its role as a meal-time glucose regulator . Stability studies have shown that nateglinide is stable under various conditions, but it undergoes extensive metabolism in the liver . Long-term studies indicate that nateglinide maintains its efficacy in regulating postprandial glucose levels without significant degradation .
Dosage Effects in Animal Models
In animal models, the effects of nateglinide vary with dosage. Studies have shown that higher doses of nateglinide result in increased insulin secretion and more significant reductions in blood glucose levels . At very high doses, nateglinide can cause hypoglycemia and other adverse effects . The optimal dosage for achieving desired glucose control without adverse effects has been identified through pharmacokinetic and pharmacodynamic studies .
Metabolic Pathways
Nateglinide is extensively metabolized in the liver, primarily by the cytochrome P450 enzymes CYP2C9 and CYP3A4 . It undergoes biotransformation to at least nine metabolites, with the major metabolites being hydroxylation products . The predominant metabolite, M1, is monohydroxylated at the methane carbon . These metabolites have significantly less antidiabetic activity compared to nateglinide . The drug is excreted predominantly in urine, with only a small fraction excreted unchanged .
Transport and Distribution
Nateglinide is rapidly absorbed from the gastrointestinal tract and reaches peak plasma concentrations quickly after oral administration . It is highly bound to plasma proteins, which facilitates its distribution throughout the body . Nateglinide is transported across the intestinal brush-border membrane via a proton-coupled transport system distinct from PepT1 and MCT1 . This transport mechanism ensures efficient absorption and distribution of nateglinide within the body .
Subcellular Localization
Nateglinide’s subcellular localization is primarily within the pancreatic beta cells, where it exerts its insulinotropic effects . The drug targets ATP-sensitive potassium channels on the cell membrane, leading to localized depolarization and calcium influx . This targeted action ensures that nateglinide effectively stimulates insulin release in response to elevated blood glucose levels . The subcellular localization of nateglinide is crucial for its role in regulating postprandial glucose levels .
Métodos De Preparación
Synthetic Routes and Reaction Conditions: Nateglinide is synthesized from R-phenylalanine and trans-4-isopropyl cyclohexane carboxylic acid. The synthesis involves the formation of an amide bond between the amino group of R-phenylalanine and the carboxyl group of trans-4-isopropyl cyclohexane carboxylic acid . The reaction typically requires the use of coupling reagents such as dicyclohexylcarbodiimide (DCC) and 1-hydroxybenzotriazole (HOBt) to facilitate the formation of the amide bond.
Industrial Production Methods: In industrial settings, Nateglinide can be produced using a similar synthetic route but on a larger scale. The process involves the use of large reactors and precise control of reaction conditions to ensure high yield and purity of the final product. The use of automated systems for monitoring and controlling the reaction parameters is common in industrial production .
Análisis De Reacciones Químicas
Types of Reactions: Nateglinide undergoes various chemical reactions, including:
Oxidation: Nateglinide can be oxidized to form its corresponding sulfoxide and sulfone derivatives.
Reduction: Reduction of Nateglinide can lead to the formation of its reduced amine derivative.
Substitution: Nateglinide can undergo nucleophilic substitution reactions, particularly at the amide nitrogen.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents such as hydrogen peroxide or m-chloroperbenzoic acid (m-CPBA) can be used.
Reduction: Reducing agents like lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4) are typically employed.
Substitution: Nucleophiles such as amines or thiols can be used under basic conditions to facilitate substitution reactions.
Major Products:
Oxidation Products: Sulfoxide and sulfone derivatives.
Reduction Products: Reduced amine derivative.
Substitution Products: Various substituted amides depending on the nucleophile used.
Comparación Con Compuestos Similares
Nateglinide is often compared with other meglitinides and antidiabetic agents, such as:
Repaglinide: Another meglitinide with a similar mechanism of action but a longer duration of action.
Metformin: A biguanide that improves insulin sensitivity and reduces hepatic glucose production.
Nateglinide’s uniqueness lies in its rapid onset and short duration of action, making it particularly effective in controlling postprandial blood glucose levels without significantly increasing the risk of hypoglycemia .
Propiedades
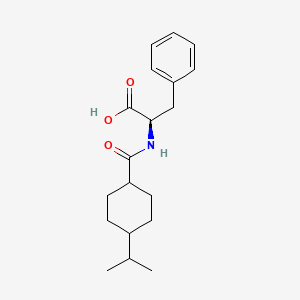
IUPAC Name |
(2R)-3-phenyl-2-[(4-propan-2-ylcyclohexanecarbonyl)amino]propanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H27NO3/c1-13(2)15-8-10-16(11-9-15)18(21)20-17(19(22)23)12-14-6-4-3-5-7-14/h3-7,13,15-17H,8-12H2,1-2H3,(H,20,21)(H,22,23)/t15?,16?,17-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OELFLUMRDSZNSF-OFLPRAFFSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C1CCC(CC1)C(=O)NC(CC2=CC=CC=C2)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)C1CCC(CC1)C(=O)N[C@H](CC2=CC=CC=C2)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H27NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID9040687 | |
| Record name | Nateglinide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9040687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
317.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Practically insoluble | |
| Record name | Nateglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00731 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Nateglinide activity is dependent on the presence functioning β cells and glucose. In contrast to sulfonylurea insulin secretatogogues, nateglinide has no effect on insulin release in the absence of glucose. Rather, it potentiates the effect of extracellular glucose on ATP-sensitive potassium channel and has little effect on insulin levels between meals and overnight. As such, nateglinide is more effective at reducing postprandial blood glucose levels than fasting blood glucose levels and requires a longer duration of therapy (approximately one month) before decreases in fasting blood glucose are observed. The insulinotropic effects of nateglinide are highest at intermediate glucose levels (3 to 10 mmol/L) and it does not increase insulin release already stimulated by high glucose concentrations (greater than 15 mmol/L). Nateglinide appears to be selective for pancreatic β cells and does not appear to affect skeletal or cardiac muscle or thyroid tissue. | |
| Record name | Nateglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00731 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
105816-04-4, 105816-06-6 | |
| Record name | Nateglinide [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0105816044 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | N-((cis-4-(1-Methylethyl)cyclohexyl)carbonyl)-D-phenylalanine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0105816066 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Nateglinide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00731 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nateglinide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9040687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | NATEGLINIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/41X3PWK4O2 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | N-((CIS-4-(1-METHYLETHYL)CYCLOHEXYL)CARBONYL)-D-PHENYLALANINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/XTM4DQP5S5 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of nateglinide?
A: Nateglinide exerts its hypoglycemic effect by selectively and reversibly inhibiting pancreatic beta-cell ATP-sensitive potassium (KATP) channels. [, , ]
Q2: How does nateglinide's interaction with KATP channels differ from that of sulfonylureas?
A: While both nateglinide and sulfonylureas bind to the sulfonylurea receptor 1 (SUR1) subunit of KATP channels, nateglinide exhibits rapid association and dissociation kinetics, leading to a faster onset and shorter duration of action. [, ]
Q3: What are the downstream effects of nateglinide binding to KATP channels?
A: Binding of nateglinide to SUR1 closes KATP channels, leading to membrane depolarization, opening of voltage-gated calcium channels, and ultimately, increased intracellular calcium levels. This rise in intracellular calcium triggers the release of insulin from pancreatic beta-cells. [, , ]
Q4: Does nateglinide have any effects on incretin hormones?
A: Research suggests that nateglinide may also stimulate the release of glucagon-like peptide-1 (GLP-1) from intestinal L cells, contributing to its glucose-lowering effects. []
Q5: What is the molecular formula and weight of nateglinide?
A5: The molecular formula of nateglinide is C19H27NO3, and its molecular weight is 317.42 g/mol.
Q6: Are there any spectroscopic data available for nateglinide?
A: Several studies employed spectroscopic techniques to characterize nateglinide and its formulations. Fourier transform infrared spectroscopy (FTIR) was used to investigate drug-excipient compatibility and confirm the formation of inclusion complexes. [, , ]
Q7: What are the common excipients used in nateglinide formulations?
A: Studies evaluated the compatibility of nateglinide with various excipients, including β-cyclodextrin (β-CD), hydroxypropyl-β-cyclodextrin (HPCD), meglumine, chitosan, ethyl cellulose, polyvinyl alcohol, and sodium tripolyphosphate. [, , , ]
Q8: How does the choice of excipients affect the stability and dissolution of nateglinide formulations?
A: Inclusion complexes of nateglinide with β-CD and HPCD showed enhanced solubility and dissolution rates compared to the free drug. [, ] Dry co-grinding with meglumine also improved dissolution and hypoglycemic effects in diabetic rats. []
Q9: How is nateglinide absorbed and distributed in the body?
A: Nateglinide is rapidly absorbed after oral administration, reaching peak plasma concentrations within 1 hour. Food intake can affect the rate but not the extent of absorption. [, ]
Q10: What are the primary metabolic pathways of nateglinide?
A: Nateglinide is primarily metabolized by cytochrome P450 (CYP) enzymes, mainly CYP2C9 and CYP3A4, into several metabolites, with M7 being the major metabolite. [, ]
Q11: How is nateglinide eliminated from the body?
A: Nateglinide is predominantly eliminated through hepatic metabolism, with a small fraction excreted unchanged in the urine. [, ]
Q12: Does renal impairment affect nateglinide pharmacokinetics?
A: Studies suggest that renal impairment, even in patients undergoing hemodialysis, does not significantly affect the pharmacokinetics of nateglinide, and dose adjustment is not required in these patients. []
Q13: How is the efficacy of nateglinide evaluated in preclinical settings?
A: Animal models, like Goto-Kakizaki (GK) rats, have been used to investigate the pharmacokinetics and pharmacodynamics of nateglinide. []
Q14: What are the key findings from clinical trials evaluating nateglinide efficacy?
A: Clinical trials demonstrated that nateglinide effectively reduces postprandial glucose excursions and improves glycemic control in patients with type 2 diabetes. Both monotherapy and combination therapy with metformin have shown efficacy. [, , , , , ]
Q15: How does the efficacy of nateglinide compare to other antidiabetic agents?
A: Head-to-head clinical trials compared nateglinide with other antidiabetic drugs like acarbose and glimepiride. Results showed comparable efficacy in reducing HbA1c levels, although differences in postprandial glucose control and effects on lipid profiles were observed. [, , , , ]
Q16: What is the solubility of nateglinide in various media?
A: Nateglinide exhibits low solubility in water. Studies have explored methods to enhance its solubility, such as the formation of inclusion complexes with cyclodextrins. [, ]
Q17: How do different formulation strategies affect the dissolution rate of nateglinide?
A: Dry co-grinding with meglumine and complexation with cyclodextrins significantly enhanced the dissolution rate of nateglinide, potentially improving its bioavailability. [, , ]
Q18: What analytical techniques are commonly used to quantify nateglinide in biological samples?
A: High-performance liquid chromatography (HPLC) coupled with various detection methods, such as UV and mass spectrometry (MS), is widely used for the quantification of nateglinide in biological matrices. [, , , ]
Q19: Have any analytical methods for nateglinide been validated?
A: Several studies reported the development and validation of analytical methods for nateglinide quantification, ensuring accuracy, precision, specificity, and linearity for research and quality control purposes. [, , , ]
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![(2R)-6-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-2,3-dihydro-1,4-benzodioxine-2-carboxylic acid](/img/structure/B1676890.png)
![(15S,16S,18S)-16-Hydroxy-15-methyl-16-(propylaminomethyl)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.115,18.02,6.07,27.08,13.019,26.020,25]octacosa-1,6,8,10,12,20,22,24,26-nonaen-3-one](/img/structure/B1676897.png)