Piracetam
Descripción general
Descripción
Piracetam es un fármaco nootrópico que pertenece al grupo de las racetams, con el nombre químico 2-oxo-1-pirrolidina acetamida. Es un derivado cíclico del neurotransmisor ácido gamma-aminobutírico (GABA). This compound fue comercializado por primera vez por UCB Pharma en 1971 y es conocido por sus propiedades de mejora cognitiva. Se utiliza en diversos trastornos cognitivos, vértigo, mioclono cortical, dislexia y anemia de células falciformes .
Mecanismo De Acción
El mecanismo de acción de piracetam no se comprende completamente, pero se cree que implica varias vías:
Modulación de Neurotransmisores: this compound modula la neurotransmisión en los sistemas colinérgico y glutamatérgico.
Neuroprotección: Tiene propiedades neuroprotectoras, mejorando la plasticidad neuronal y protegiendo contra la hipoxia.
Efectos Vasculares: This compound reduce la adhesión de los eritrocitos al endotelio vascular, evitando los espasmos vasculares y facilitando la microcirculación .
Aplicaciones Científicas De Investigación
Piracetam tiene una amplia gama de aplicaciones de investigación científica:
Química: Se utiliza como compuesto modelo para estudiar las propiedades de las amidas cíclicas y sus derivados.
Biología: Se ha investigado por sus efectos sobre la fluidez de la membrana celular y la neuroprotección.
Medicina: Se utiliza en el tratamiento de trastornos cognitivos, mioclono y anemia de células falciformes. También se ha estudiado por sus posibles beneficios en afecciones como la demencia, el vértigo y la dislexia.
Industria: Se utiliza en el desarrollo de suplementos nootrópicos y potenciadores cognitivos .
Análisis Bioquímico
Biochemical Properties
Piracetam interacts with various enzymes, proteins, and other biomolecules. It shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid and is a cyclic derivative of the neurotransmitter γ-aminobutyric acid (GABA) . Its mechanism of action differs from that of endogenous GABA . This compound has neuroprotective and anticonvulsant properties and is reported to improve neural plasticity .
Cellular Effects
This compound has significant effects on various types of cells and cellular processes. It enhances the growth of cells, inhibits oxidative stress, and improves mitochondrial function . It influences cell function by modulating neurotransmission in a range of transmitter systems, including cholinergic and glutamatergic systems .
Molecular Mechanism
This compound exerts its effects at the molecular level through various mechanisms. It influences neuronal and vascular functions by restoring cell membrane fluidity . This mechanism of action is thought to improve membrane stability, allowing the membrane and transmembrane proteins to maintain and recover the three-dimensional structure or folding for normal function .
Temporal Effects in Laboratory Settings
This compound shows changes in its effects over time in laboratory settings. It has been observed that this compound increases regional cerebral blood flow . This suggests that this compound might have long-term effects on cellular function observed in in vitro or in vivo studies .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. For instance, daily this compound treatment at doses of 0, 75, 150, and 300 mg/kg ip was initiated in 6-week-old male mice. The study found that low doses of this compound reduced search time in the visible-platform component, while all this compound doses prevented trial-related improvements in performance .
Metabolic Pathways
This compound is involved in various metabolic pathways. It has been shown to alter the physical properties of the plasma membrane by increasing its fluidity and protecting the cell against hypoxia . It also increases red cell deformability and normalizes the aggregation of hyperactive platelets .
Transport and Distribution
This compound is transported and distributed within cells and tissues. After oral ingestion, this compound is rapidly absorbed with a bioavailability of 100%. Its volume of distribution is 0.6 L/kg and plasma protein binding is 0% .
Subcellular Localization
It is known that this compound influences neuronal and vascular functions by restoring cell membrane fluidity . This suggests that this compound might have specific targeting signals or post-translational modifications that direct it to specific compartments or organelles.
Métodos De Preparación
Rutas Sintéticas y Condiciones de Reacción
Piracetam se puede sintetizar mediante varios métodos. Un método común implica la reacción de 2-pirrolidona con cloroacetato de etilo en presencia de una base, seguido de hidrólisis para producir this compound. Otro método implica la ciclización del ácido gamma-aminobutírico (GABA) con anhídrido acético .
Métodos de Producción Industrial
La producción industrial de this compound generalmente implica la síntesis a gran escala utilizando los métodos mencionados anteriormente. El proceso está optimizado para un alto rendimiento y pureza, asegurando que el producto final cumpla con los estándares farmacéuticos. El proceso de producción incluye pasos como la cristalización, la filtración y el secado para obtener el compuesto puro .
Análisis De Reacciones Químicas
Tipos de Reacciones
Piracetam experimenta diversas reacciones químicas, incluyendo:
Oxidación: this compound se puede oxidar para formar ácido 2-pirrolidona-5-carboxílico.
Reducción: La reducción de this compound puede producir 2-pirrolidona.
Sustitución: Las reacciones de sustitución pueden ocurrir en el grupo acetamida, lo que lleva a la formación de varios derivados
Reactivos y Condiciones Comunes
Oxidación: Los agentes oxidantes comunes incluyen permanganato de potasio y peróxido de hidrógeno.
Reducción: Se pueden utilizar agentes reductores como el hidruro de litio y aluminio.
Sustitución: Los reactivos como los haluros de alquilo y los cloruros de acilo se utilizan comúnmente para reacciones de sustitución
Principales Productos Formados
Oxidación: Ácido 2-pirrolidona-5-carboxílico
Reducción: 2-pirrolidona
Sustitución: Diversos derivados de la acetamida
Comparación Con Compuestos Similares
Piracetam a menudo se compara con otras racetams, incluyendo:
Oxiracetam: Conocido por sus efectos estimulantes y utilizado para la mejora cognitiva.
Aniracetam: Tiene propiedades ansiolíticas y se utiliza para la ansiedad y los trastornos cognitivos.
Pramiracetam: Más potente que el this compound, utilizado para los déficits cognitivos asociados con lesiones cerebrales traumáticas.
Fenilthis compound: Más potente y utilizado para una gama más amplia de indicaciones, incluyendo la mejora cognitiva y el rendimiento físico .
This compound es único por su perfil de seguridad bien documentado y su amplia gama de aplicaciones, lo que lo convierte en un compuesto versátil tanto en entornos de investigación como clínicos.
Propiedades
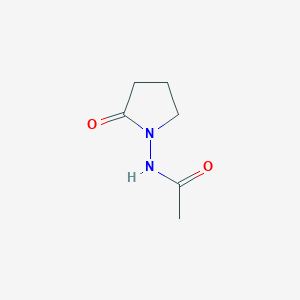
IUPAC Name |
2-(2-oxopyrrolidin-1-yl)acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C6H10N2O2/c7-5(9)4-8-3-1-2-6(8)10/h1-4H2,(H2,7,9) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GMZVRMREEHBGGF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC(=O)N(C1)CC(=O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C6H10N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5044491 | |
| Record name | 2-Oxo-1-Pyrrolidineacetamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5044491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
142.16 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Boiling Point |
Decomposes | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Piracetam interacts with the polar heads in the phospholipids membrane and the resulting mobile drug-lipid complexes are thought to reorganize the lipids and influence membrane function and fluidity. Such interaction has been reported in a study that investigated the effects of neuronal outgrowth induced by beta amyloid peptides; while amyloid peptides cause lipid disorganization within the cell membranes leading to neuronal death, piracetam demonstrated to decrease the destabilizing effects of amyloid peptide. The authors suggest that piracetam induces a positive curvature of the membrane by occupying the polar groups in the phospholipids to counteract the negative curvature induced by amyloid peptides , which in turn would decrease the likelihood of membrane fusion. This mechanism of action is thought to improve membrane stability, allowing the membrane and transmembrane proteins to maintain and recover the three-dimensional structure or folding for normal function such as membrane transport, chemical secretion, and receptor binding and stimulation. Through restored membrane fluidity, piracetam promotes restored neurotransmission such as glutamatergic and cholinergic systems, enhances neuroplasticity and mediates neuroprotective and anticonvulsant effects at the neuronal level. It is also demonstrated that piracetam also improves the fluidity of platelet membranes. At the vascular level, piracetam decreases adhesion of erythrocytes to cell wall and reduces vasospasm which in turn improves microcirculation including cerebral and renal blood flow., It was found that a drug of the nootropic nature piracetam possessing pronounced antihypoxic properties eliminates calcium chloride-induced disturbances of the cardiac rhythm and significantly raises the threshold of atrial fibrillation during electrical stimulation. The drug's antiarrhythmic effect is followed by a decrease of the rhythm rate and an increase of the contraction amplitude. The animals treated with piracetam in a dose when its antiarrhythmic effects (300 mg/kg) exhibited a decrease of the membrane potential of erythrocytes as compared with control. Similar effects occurred in the animals treated with lidocaine. It can be concluded that in certain types of arrhythmias the use of piracetam restores the normal rhythm of contractions that is perhaps connected with its positive influence on metabolic processes in the myocardium. | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PIRACETAM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from isopropanol | |
CAS No. |
7491-74-9 | |
| Record name | Piracetam | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=7491-74-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Piracetam [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007491749 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | piracetam | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758191 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 2-Oxo-1-Pyrrolidineacetamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5044491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Piracetam | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.028.466 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PIRACETAM | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/ZH516LNZ10 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PIRACETAM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
151.5 - 152.5 °C | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PIRACETAM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![[1]Benzothieno[3,2-d]pyrimidin-4(3H)-one, 2-[(dimethylamino)methyl]-8-(4-hydroxyphenyl)-](/img/structure/B1677885.png)