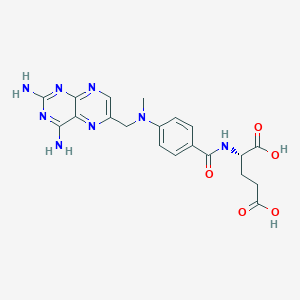
Metotrexato
Descripción general
Descripción
Metotrexato, anteriormente conocido como ametopterina, es un agente quimioterapéutico e inmunosupresor. Se utiliza ampliamente para tratar varios tipos de cáncer, enfermedades autoinmunes y embarazos ectópicos. El compuesto es particularmente efectivo contra el cáncer de mama, la leucemia, el cáncer de pulmón, el linfoma, la enfermedad trofoblástica gestacional y el osteosarcoma . Además, se utiliza para controlar afecciones autoinmunes como la psoriasis, la artritis reumatoide y la enfermedad de Crohn .
Mecanismo De Acción
El metotrexato ejerce sus efectos al inhibir varias enzimas responsables de la síntesis de nucleótidos, incluida la dihidrofolato reductasa, la timidilato sintasa y la aminoimidazol carboxamida ribonucleótido transformilasa . Esta inhibición conduce a la supresión de la síntesis de ADN y la división celular, lo que la hace efectiva contra las células cancerosas que se dividen rápidamente . Además, el this compound promueve la liberación de adenosina, que tiene efectos antiinflamatorios .
Aplicaciones Científicas De Investigación
El metotrexato tiene una amplia gama de aplicaciones en la investigación científica:
Análisis Bioquímico
Biochemical Properties
Methotrexate plays a significant role in biochemical reactions. It interacts with enzymes, proteins, and other biomolecules. For instance, it is known to inhibit the enzyme dihydrofolate reductase (DHFR), which is involved in the synthesis of tetrahydrofolate, a cofactor required for the synthesis of nucleotides .
Cellular Effects
Methotrexate has profound effects on various types of cells and cellular processes. It influences cell function by impacting cell signaling pathways, gene expression, and cellular metabolism. For example, by inhibiting DHFR, Methotrexate disrupts the synthesis of nucleotides, thereby affecting DNA replication and cell division .
Molecular Mechanism
Methotrexate exerts its effects at the molecular level through binding interactions with biomolecules, enzyme inhibition, and changes in gene expression. Its primary mechanism of action is the competitive inhibition of DHFR, which leads to a decrease in the synthesis of nucleotides and subsequently affects DNA replication .
Temporal Effects in Laboratory Settings
The effects of Methotrexate change over time in laboratory settings. Information on the product’s stability, degradation, and any long-term effects on cellular function observed in in vitro or in vivo studies is crucial. For instance, a study using high-performance liquid chromatography–selected reaction monitoring– mass spectrometry (HPLC-SRM-MS) based biochemical analysis of drug levels was conducted to monitor Methotrexate adherence .
Dosage Effects in Animal Models
The effects of Methotrexate vary with different dosages in animal models. High doses of Methotrexate can lead to toxic or adverse effects. The specific threshold effects observed in these studies are beyond the scope of this article .
Metabolic Pathways
Methotrexate is involved in various metabolic pathways. It interacts with enzymes or cofactors, and it can affect metabolic flux or metabolite levels. For example, by inhibiting DHFR, Methotrexate disrupts the folate metabolic pathway .
Transport and Distribution
Methotrexate is transported and distributed within cells and tissues. It can interact with various transporters or binding proteins, and it can affect its localization or accumulation .
Subcellular Localization
The subcellular localization of Methotrexate and its effects on its activity or function are crucial. Methotrexate primarily localizes in the cytoplasm where it exerts its inhibitory effects on DHFR .
Métodos De Preparación
Rutas Sintéticas y Condiciones de Reacción: El metotrexato se sintetiza mediante un proceso de varios pasos que implica la reacción del ácido 4-aminobenzoico con 2,4-diamino-6-hidroxipirimidina para formar ácido 4-amino-4-desoxi-N10-metilpteroico. Este intermedio se acopla luego con ácido L-glutámico para producir this compound .
Métodos de Producción Industrial: La producción industrial de this compound implica la síntesis a gran escala utilizando condiciones de reacción similares a las de la síntesis de laboratorio, pero optimizadas para obtener mayores rendimientos y pureza. El proceso incluye pasos de purificación rigurosos como la cristalización y la cromatografía para garantizar que el producto final cumpla con los estándares farmacéuticos .
Análisis De Reacciones Químicas
Tipos de Reacciones: El metotrexato sufre varias reacciones químicas, incluida la oxidación, la reducción y la sustitución.
Reactivos y Condiciones Comunes:
Oxidación: El this compound se puede oxidar usando agentes como el peróxido de hidrógeno en condiciones ácidas.
Reducción: Las reacciones de reducción implican el uso de agentes reductores como el borohidruro de sodio.
Sustitución: Las reacciones de sustitución pueden ocurrir con nucleófilos como las aminas en condiciones básicas.
Productos Principales: Los principales productos formados a partir de estas reacciones incluyen 7-hidroxithis compound y otros metabolitos que retienen alguna actividad biológica .
Comparación Con Compuestos Similares
El metotrexato es estructuralmente similar al ácido fólico y la aminopterina. Es único en su forma N-metil, que aumenta su potencia como antimetabolito . Compuestos similares incluyen:
Aminopterina: Un agente antifolato anterior con mecanismos similares pero mayor toxicidad.
Pemetrexed: Otro antifolato utilizado en el tratamiento del cáncer, con un espectro de actividad más amplio.
Raltitrexed: Un inhibidor de la timidilato sintasa utilizado en el cáncer colorrectal.
La estructura única y el mecanismo de acción del this compound lo convierten en un valioso agente terapéutico con diversas aplicaciones en medicina e investigación.
Propiedades
IUPAC Name |
(2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methylamino]benzoyl]amino]pentanedioic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FBOZXECLQNJBKD-ZDUSSCGKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)NC(CCC(=O)O)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)N[C@@H](CCC(=O)O)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H22N8O5 | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
15475-56-6 (hydrochloride salt), 7413-34-5 (di-hydrochloride salt) | |
| Record name | Methotrexate [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000059052 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID4020822 | |
| Record name | Methotrexate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4020822 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
454.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Methotrexate is an odorless yellow to orange-brown crystalline powder. (NTP, 1992) It is a chemotherapy drug that interferes with DNA and RNA synthesis., Solid, Bright yellow-orange, odorless, crystalline powder. | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methotrexate | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014703 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | METHOTREXATE | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/874 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
Solubility |
less than 1 mg/mL at 66 °F (NTP, 1992), Insoluble in water , alcohol, chloroform, ether; slightly soluble in dilute hydrochloric acid; soluble in dil solns of alkali hydroxides and carbonates., Practically insoluble in water and in alcohol., In water, 2.6X10+3 mg/L at 25 °C /Estimated/, 1.71e-01 g/L | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Methotrexate | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014703 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
2.1X10-19 mm Hg at 25 °C /Estimated/ | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Methotrexate enters tissues and is converted to a methotrexate polyglutamate by folylpolyglutamate. Methotrexate's mechanism of action is due to its inhibition of enzymes responsible for nucleotide synthesis including dihydrofolate reductase, thymidylate synthase, aminoimidazole caboxamide ribonucleotide transformylase (AICART), and amido phosphoribosyltransferase. Inhibtion of nucleotide synthesis prevents cell division. In rheumatoid arthritis, methotrexate polyglutamates inhibit AICART more than methotrexate. This inhibition leads to accumulation of AICART ribonucleotide, which inhibits adenosine deaminase, leading to an accumulation of adenosine triphosphate and adenosine in the extracellular space, stimulating adenosine receptors, leading to anti-inflammatory action., Methotrexate and its polyglutanate metabolites reversibly inhibits dihydrofolate reductase, the enzyme that reduces folic acid to tetrahydrofolic acid. Inhibition of tetrahydrofolate formation limits the availability of one-carbon fragments necessary for synthesis of purines and the conversion of deoxyuridylate to thymidylate in the synthesis of DNA and cell reproduction. The affinity of dihydrofolate reductase for methotrexate is far greater than its affinity for folic acid or dihydrofolic acid. and, therefore, even very large doses of folic acid given simultaneously will not reverse the effects of methotrexate. Leucovorin calcium, a derivative of tetrahydrofolic acid, may block the effects of methotrexate if given shortly after the antineoplastic agent. Results of one study indicate that methotrexate also causes an increase in intracellular deoxyadenosine triphosphate, which is thought to inhibit ribonucleotide reduction, and polynucleotide ligase, an enzyme concerned in DNA synthesis and repair. Tissues with high rates of cellular proliferation such as neoplasms, psoriatic epidermis, bone marrow, the lining of the GI tract, hair matrix, and fetal cells are most sensitive to the effects of methotrexate., Methotrexate ... has immunosuppressive activity, in part possibly as a result of inhibition of lymphocyte multiplication. The mechanism(s) of action in the management of rheumatoid arthritis of the drug is not known, although suggested mechanisms have included immunosuppressive and/or antiinflammatory effects. | |
| Record name | Methotrexate | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00563 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Orange-brown, crystalline powder | |
CAS No. |
59-05-2 | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methotrexate | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=59-05-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Methotrexate [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000059052 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Methotrexate | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00563 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | methotrexate | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=740 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | L-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Methotrexate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4020822 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Methotrexate | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.376 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | METHOTREXATE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/YL5FZ2Y5U1 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Methotrexate | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014703 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | METHOTREXATE | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/874 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
Melting Point |
365 to 399 °F (decomposes) (NTP, 1992), Melting point: 185-204 °C. /Methotrexate monohydrate/, 195 °C, 356-399 °F | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methotrexate | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00563 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Methotrexate | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014703 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | METHOTREXATE | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/874 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![(2S,3S)-3-amino-4-(3,3-difluoropyrrolidin-1-yl)-N,N-dimethyl-4-oxo-2-[4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)phenyl]butanamide;hydrochloride](/img/structure/B535588.png)
![4-[2-(3,4-dimethylanilino)-1,3-thiazol-4-yl]-N,N-diethylbenzenesulfonamide](/img/structure/B535868.png)
![5-Chloro-6-[(2,5-dimethoxyanilino)methyl]quinazoline-2,4-diamine](/img/structure/B535878.png)
![N-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-{[5-(3,4,5-trimethoxyphenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide](/img/structure/B536827.png)
![4-[1-Butyl-4-(4-chlorophenyl)-4-hydroxypiperidin-1-ium-1-yl]-1-(4-fluorophenyl)butan-1-one;iodide](/img/structure/B537403.png)
![N,N'-bis[[1-amino-3-(1,3-dioxoisoindol-2-yl)propylidene]amino]pentanediamide](/img/structure/B537568.png)
![3-(5-methyl-7-oxo-3-phenylfuro[3,2-g]chromen-6-yl)-N-(oxolan-2-ylmethyl)propanamide](/img/structure/B537580.png)