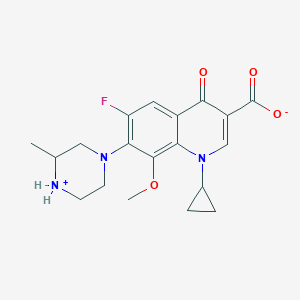
Gatifloxacine
Vue d'ensemble
Description
- Quetiapine Fumarate, also known by its brand names Seroquel and Seroquel XR, belongs to the class of medications called atypical antipsychotics.
- It is primarily used to treat several mental and mood disorders, including schizophrenia, bipolar disorder, and major depressive disorder.
- Quetiapine works by modulating the activity of certain neurotransmitters in the brain, specifically serotonin and dopamine .
Applications De Recherche Scientifique
- In chemistry, quetiapine serves as a valuable tool for studying neurotransmitter interactions and receptor binding.
- In biology, it aids in understanding brain function and the role of neurotransmitters in mental health.
- In medicine, it is crucial for managing schizophrenia, bipolar disorder, and depression.
- In industry, quetiapine contributes to the development of antipsychotic medications.
Mécanisme D'action
Target of Action
Gatifloxacin, a member of the fourth-generation fluoroquinolone family, primarily targets bacterial enzymes DNA gyrase and topoisomerase IV . These enzymes play a crucial role in bacterial DNA replication, transcription, repair, and recombination .
Mode of Action
Gatifloxacin inhibits its primary targets, DNA gyrase and topoisomerase IV . This inhibition prevents the unwinding and supercoiling of bacterial DNA, which are essential steps in DNA replication, transcription, repair, and recombination . As a result, the bacterial cell cannot replicate its DNA, leading to cell death .
Biochemical Pathways
The bactericidal action of Gatifloxacin results from the disruption of DNA replication and transcription processes in bacteria . By inhibiting DNA gyrase and topoisomerase IV, Gatifloxacin prevents the unwinding and supercoiling of bacterial DNA, which are necessary for these processes . This disruption leads to the cessation of essential cellular functions and ultimately results in bacterial cell death .
Pharmacokinetics
Gatifloxacin exhibits high oral bioavailability (96%), allowing oral and intravenous formulations to be bioequivalent and interchangeable . It has a large volume of distribution (~1.8 L/kg), low protein binding (~20%), and broad tissue distribution . Gatifloxacin is primarily excreted unchanged in the urine (>80%) . Its pharmacokinetics are linear and time-independent at doses ranging from 200 to 800 mg, administered over a period of up to 14 days .
Result of Action
The inhibition of DNA gyrase and topoisomerase IV by Gatifloxacin leads to the prevention of bacterial DNA replication, transcription, repair, and recombination . This results in the cessation of essential cellular functions, leading to bacterial cell death . Therefore, Gatifloxacin is effective in treating a wide variety of infections in the body .
Action Environment
The efficacy and stability of Gatifloxacin can be influenced by various environmental factors. It’s important to note that indiscriminate use of Gatifloxacin, like other antibiotics, must be avoided to prevent the potential for selection of widespread resistance .
Analyse Biochimique
Biochemical Properties
Gatifloxacin works by inhibiting the bacterial enzymes DNA gyrase and topoisomerase IV . These enzymes are required for bacterial DNA replication, transcription, repair, and recombination . By inhibiting these enzymes, Gatifloxacin prevents bacteria from replicating, thereby stopping the infection.
Cellular Effects
Gatifloxacin’s primary cellular effect is the inhibition of bacterial DNA gyrase and topoisomerase IV enzymes . This inhibition disrupts the bacterial DNA replication process, leading to cell death . Gatifloxacin is effective against a broad spectrum of bacteria, including Gram-positive, Gram-negative, and anaerobic bacteria .
Molecular Mechanism
The bactericidal action of Gatifloxacin results from the inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV . These enzymes are essential for bacterial DNA replication, transcription, repair, and recombination . By inhibiting these enzymes, Gatifloxacin prevents the bacteria from replicating their DNA, leading to cell death .
Temporal Effects in Laboratory Settings
Gatifloxacin demonstrates improved in vitro activity against gram-positive organisms, pharmacokinetics, and pharmacodynamics when compared with levofloxacin and ciprofloxacin
Dosage Effects in Animal Models
It’s primarily excreted unchanged in the urine .
Metabolic Pathways
Gatifloxacin undergoes limited biotransformation in humans with less than 1% of the dose excreted in the urine as ethylenediamine and methylethylenediamine metabolites . It does not inhibit the cytochrome P450 (CYP) system and thus is not expected to interfere with CYP-dependent metabolism of drugs .
Transport and Distribution
Gatifloxacin has a large volume of distribution, low protein binding, and broad tissue distribution . It’s primarily excreted unchanged in the urine
Méthodes De Préparation
- Quetiapine Fumarate can be synthesized through various routes, but one common method involves the reaction of quetiapine base with fumaric acid.
- Industrial production methods typically involve large-scale chemical synthesis, ensuring high purity and yield.
Analyse Des Réactions Chimiques
- Quetiapine undergoes several reactions, including oxidation, reduction, and substitution.
- Common reagents and conditions used in these reactions include strong acids, bases, and reducing agents.
- Major products formed include quetiapine fumarate itself and its metabolites.
Comparaison Avec Des Composés Similaires
- Quetiapine stands out due to its dual action on serotonin and dopamine receptors.
- Similar compounds include other atypical antipsychotics like risperidone and olanzapine.
Propriétés
IUPAC Name |
1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-4-ium-1-yl)-4-oxoquinoline-3-carboxylate | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H22FN3O4/c1-10-8-22(6-5-21-10)16-14(20)7-12-15(18(16)27-2)23(11-3-4-11)9-13(17(12)24)19(25)26/h7,9-11,21H,3-6,8H2,1-2H3,(H,25,26) | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XUBOMFCQGDBHNK-UHFFFAOYSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1CN(CC[NH2+]1)C2=C(C=C3C(=C2OC)N(C=C(C3=O)C(=O)[O-])C4CC4)F | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H22FN3O4 | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
375.4 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Gatifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015178 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
6.31e-01 g/L | |
| Record name | Gatifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015178 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
112811-59-3 | |
| Record name | Gatifloxacin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=112811-59-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Gatifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015178 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
182 - 185 °C | |
| Record name | Gatifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015178 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of gatifloxacin?
A1: Gatifloxacin, an 8-methoxyfluoroquinolone, primarily targets bacterial DNA gyrase and topoisomerase IV. [] These enzymes are essential for bacterial DNA replication and repair. Gatifloxacin inhibits these enzymes, leading to bacterial cell death. []
Q2: How does the presence of the 8-methoxy group in gatifloxacin impact its activity compared to other fluoroquinolones?
A2: The 8-methoxy group enhances gatifloxacin's activity against Gram-positive bacteria, including those resistant to other fluoroquinolones. [] It requires mutations in both topoisomerase IV and DNA gyrase for resistance to develop, unlike some other fluoroquinolones. []
Q3: What makes gatifloxacin effective against methicillin-resistant Staphylococcus aureus (MRSA)?
A3: Gatifloxacin, particularly when combined with benzalkonium chloride (BAK), exhibits potent activity against MRSA, even at low concentrations. [] The addition of BAK significantly lowers the minimum inhibitory concentration (MIC) and mutant prevention concentration (MPC) of gatifloxacin against MRSA. [] This suggests a synergistic effect between these compounds.
Q4: What is the chemical formula and molecular weight of gatifloxacin?
A4: Unfortunately, the provided research abstracts do not explicitly state the chemical formula and molecular weight of gatifloxacin. This information would need to be sourced from a drug database or chemical reference.
Q5: How does the stability of gatifloxacin for injection vary between different manufacturers?
A5: A study evaluating four different manufacturers of gatifloxacin for injection found that all products remained stable for at least two years, meeting the requirements outlined in the Chinese Pharmacopoeia. [] This suggests consistent manufacturing processes and adherence to quality standards across different manufacturers.
Q6: How does the formulation of gatifloxacin as an ophthalmic gel affect its bioavailability compared to eye drops?
A6: Gatifloxacin ophthalmic gel demonstrates significantly increased bioavailability and a longer duration of action in the eye compared to gatifloxacin eye drops. [] The gel formulation likely enhances drug retention on the ocular surface, resulting in prolonged drug exposure.
Q7: What are the pharmacokinetic properties of gatifloxacin in infants and children?
A7: Studies in pediatric patients aged 6 months to 16 years showed that gatifloxacin, administered as an oral suspension, exhibits age-dependent pharmacokinetics. [] The apparent clearance of gatifloxacin decreases with increasing age. [] A dose of 10 mg/kg every 24 hours is expected to achieve therapeutic concentrations in this population. []
Q8: How does the co-administration of gatifloxacin with rifampicin affect its pharmacokinetics?
A8: When administered with rifampicin, isoniazid, and pyrazinamide in a tuberculosis treatment regimen, gatifloxacin's exposure initially increases due to drug interactions, but then decreases with repeated daily dosing. [] This decline in exposure with multiple doses might necessitate dose adjustments to maintain therapeutic levels.
Q9: How does the area under the concentration-time curve (AUC) relate to the development of resistance to gatifloxacin in Streptococcus pneumoniae?
A9: In vitro studies using S. pneumoniae showed a clear relationship between the ratio of the free AUC to the MIC (fAUC/MIC) and the emergence of resistance. [] Higher fAUC/MIC ratios were associated with delayed development of resistance mutations in topoisomerase genes. [] Levofloxacin, at clinical doses, showed a higher likelihood of resistance development compared to the newer fluoroquinolones like gatifloxacin, gemifloxacin, and moxifloxacin. []
Q10: What are some reported adverse effects associated with gatifloxacin administration?
A11: Gatifloxacin has been associated with dysglycemic events, including both hypoglycemia and hyperglycemia. [, , , ] These effects are thought to be related to gatifloxacin potentially influencing insulin secretion. [] Other reported side effects include gastrointestinal disturbances, dizziness, and skin reactions. [, ]
Q11: What analytical techniques are commonly used to quantify gatifloxacin in biological samples?
A13: High-performance liquid chromatography (HPLC) is a widely employed technique for quantifying gatifloxacin concentrations in various matrices, including serum, vitreous humor, and aqueous humor. [, , ] The combination of HPLC with mass spectrometry (LC-MS) further enhances the sensitivity and specificity of gatifloxacin detection. []
Q12: What is the potential impact of hexavalent chromium (Cr6+) on the interaction between gatifloxacin and DNA?
A14: Studies suggest that Cr6+ can hinder the binding of gatifloxacin to DNA. [] This interference might be due to Cr6+ competing with gatifloxacin for binding sites on DNA or forming complexes with gatifloxacin, thereby reducing its availability for DNA interaction. []
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.


![(3R,4R,5R)-2-[(1S,2S,4S,6R)-4,6-Diamino-3-[(2R,3R,6S)-3-amino-6-[1-(methylamino)ethyl]oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol;sulfuric acid](/img/structure/B562.png)