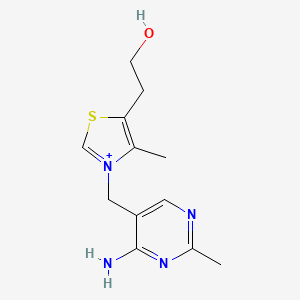
Thiamine
Vue d'ensemble
Description
La thiamine, également connue sous le nom de vitamine B1, est une vitamine hydrosoluble essentielle à la santé humaine. C'est la première vitamine B à avoir été découverte et elle joue un rôle crucial dans le métabolisme des glucides, des lipides et des protéines. La this compound se trouve dans divers aliments, notamment les céréales complètes, les légumineuses et certaines viandes et poissons. Elle est également synthétisée commercialement pour être utilisée comme complément alimentaire et médicament .
Méthodes De Préparation
Voies de synthèse et conditions de réaction : La thiamine peut être synthétisée selon plusieurs voies chimiques. Une méthode courante consiste à faire réagir le 4-méthyl-5-(2-hydroxéthyl)thiazole avec la 2-méthyl-4-amino-5-chlorométhylpyrimidine en milieu basique pour former de la this compound . La réaction nécessite généralement un solvant comme le méthanol et une base comme l'hydroxyde de sodium.
Méthodes de production industrielle : En milieu industriel, le chlorhydrate de this compound est souvent produit en faisant réagir le nitrate de this compound avec de l'acide chlorhydrique. Le procédé consiste à chauffer de l'acide chlorhydrique concentré, à refroidir et à sécher le gaz chlorure d'hydrogène désorbé, puis à l'introduire dans une solution méthanolique de nitrate de this compound. Le chlorhydrate de this compound résultant est ensuite filtré, lavé et séché .
Analyse Des Réactions Chimiques
Types de réactions : La thiamine subit diverses réactions chimiques, notamment :
Oxydation : La this compound peut être oxydée pour former du thiochrome, un composé fluorescent utilisé en chimie analytique.
Phosphorylation : La this compound est phosphorylée pour former du pyrophosphate de this compound, une coenzyme essentielle dans les réactions métaboliques.
Réactifs et conditions courants :
Oxydation : Le ferricyanure de potassium est couramment utilisé comme oxydant pour convertir la this compound en thiochrome.
Phosphorylation : La this compound est phosphorylée par la pyrophosphokinase de this compound en présence d'adénosine triphosphate (ATP).
Principaux produits :
Thiochrome : Formé par l'oxydation de la this compound.
Pyrophosphate de this compound : Formé par la phosphorylation de la this compound.
4. Applications de la recherche scientifique
La this compound a un large éventail d'applications dans la recherche scientifique :
Médecine : Utilisée pour traiter et prévenir les troubles de carence en this compound tels que le béribéri et l'encéphalopathie de Wernicke.
5. Mécanisme d'action
La this compound exerce ses effets principalement par l'intermédiaire de sa forme active, le pyrophosphate de this compound. Cette coenzyme est impliquée dans plusieurs voies métaboliques clés, notamment la décarboxylation de l'acide pyruvique et des alpha-cétoacides. Le pyrophosphate de this compound agit comme un cofacteur pour des enzymes telles que la transcétolase, la pyruvate déshydrogénase et l'alpha-cétoglutarate déshydrogénase, qui sont essentielles à la production d'énergie et à la synthèse des acides nucléiques et des acides gras .
Composés similaires :
Benfotiamine : Un dérivé liposoluble de la this compound qui présente une biodisponibilité plus élevée et est utilisé pour traiter la neuropathie diabétique.
Monophosphate de this compound : Une forme phosphorylée de la this compound qui sert d'intermédiaire dans la synthèse du pyrophosphate de this compound.
Triphosphate de this compound : Une autre forme phosphorylée de la this compound impliquée dans le métabolisme énergétique cellulaire.
Unicité : La this compound est unique parmi ses composés similaires en raison de son rôle de précurseur principal du pyrophosphate de this compound, la forme active de la coenzyme. Si la benfotiamine et d'autres dérivés ont des applications et des avantages spécifiques, la this compound elle-même est essentielle à un large éventail de processus métaboliques et est la forme la plus couramment utilisée dans les compléments alimentaires et les aliments fortifiés .
Applications De Recherche Scientifique
Thiamine has a wide range of applications in scientific research:
Medicine: Used to treat and prevent this compound deficiency disorders such as beriberi and Wernicke encephalopathy.
Industry: Added to fortified foods and dietary supplements to ensure adequate intake of this essential vitamin.
Mécanisme D'action
Thiamine exerts its effects primarily through its active form, this compound pyrophosphate. This coenzyme is involved in several key metabolic pathways, including the decarboxylation of pyruvic acid and alpha-ketoacids. This compound pyrophosphate acts as a cofactor for enzymes such as transketolase, pyruvate dehydrogenase, and alpha-ketoglutarate dehydrogenase, which are crucial for energy production and the synthesis of nucleic acids and fatty acids .
Comparaison Avec Des Composés Similaires
Benfotiamine: A lipid-soluble derivative of thiamine that has higher bioavailability and is used to treat diabetic neuropathy.
This compound Monophosphate: A phosphorylated form of this compound that serves as an intermediate in the synthesis of this compound pyrophosphate.
This compound Triphosphate: Another phosphorylated form of this compound involved in cellular energy metabolism.
Uniqueness: this compound is unique among its similar compounds due to its role as the primary precursor for this compound pyrophosphate, the active coenzyme form. While benfotiamine and other derivatives have specific applications and advantages, this compound itself is essential for a wide range of metabolic processes and is the most commonly used form in dietary supplements and fortified foods .
Propriétés
IUPAC Name |
2-[3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C12H17N4OS/c1-8-11(3-4-17)18-7-16(8)6-10-5-14-9(2)15-12(10)13/h5,7,17H,3-4,6H2,1-2H3,(H2,13,14,15)/q+1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JZRWCGZRTZMZEH-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(SC=[N+]1CC2=CN=C(N=C2N)C)CCO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C12H17N4OS+ | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID50220251 | |
| Record name | Thiamine ion | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID50220251 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
265.36 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid with a slight odor; [HSDB] Crystals; [Avocado Research MSDS], Solid | |
| Record name | Thiamine | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/17375 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
| Record name | Thiamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000235 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
500.0 mg/mL | |
| Record name | Thiamine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00152 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Thiamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000235 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
It is thought that the mechanism of action of thiamine on endothelial cells is related to a reduction in intracellular protein glycation by redirecting the glycolytic flux. Thiamine is mainly the transport form of the vitamin, while the active forms are phosphorylated thiamine derivatives. Natural derivatives of thiamine phosphate, such as thiamine monophosphate (ThMP), thiamine diphosphate (ThDP), also sometimes called thiamine pyrophosphate (TPP), thiamine triphosphate (ThTP), and thiamine triphosphate (AThTP), that act as coenzymes in addition to their each unique biological functions. | |
| Record name | Thiamine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00152 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
70-16-6, 59-43-8 | |
| Record name | 3-[(4-Amino-2-methyl-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazolium | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=70-16-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Thiamine ion | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000070166 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Thiamine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00152 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Thiamine ion | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID50220251 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Thiamine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.387 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | THIAMINE ION | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4ABT0J945J | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Thiamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000235 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
248 °C | |
| Record name | Thiamine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00152 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Thiamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000235 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.


![disodium;(6R,7R)-7-[[2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[(2-methyl-6-oxido-5-oxo-1,2,4-triazin-3-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate](/img/structure/B1217605.png)
![3,5-diacetamido-2,4,6-triiodo-N-methyl-N-[2-[methyl(2,3,4,5,6-pentahydroxyhexyl)amino]-2-oxoethyl]benzamide](/img/structure/B1217610.png)