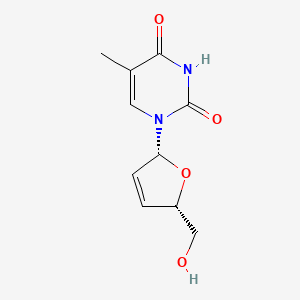
Stavudine
Vue d'ensemble
Description
La stavudine est un analogue nucléosidique inhibiteur de la transcriptase inverse (INTI) utilisé principalement dans le traitement de l'infection par le virus de l'immunodéficience humaine (VIH). Elle a été décrite pour la première fois en 1966 et approuvée pour une utilisation aux États-Unis en 1994 . La this compound agit en inhibant l'activité de la transcriptase inverse du VIH-1, une enzyme essentielle à la réplication du virus .
Mécanisme D'action
Stavudine inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA.
Enzymatic conversion of this compound to d4T-triphosphate appears to be complex, involving several steps and enzymes. This compound is first converted to dideoxydidehydrothymidine-5'-monophosphate (d4T-monophosphate) by thymidine kinase. Subsequently, d4T-monophosphate is converted to dideoxydidehydrothymidine-5'-diphosphate (d4T-diphosphate), and then to d4T-triphosphate, presumably by the same cellular kinases involved in the metabolism of zidovudine. ... d4T-Triphosphate is a structural analog of thymidine triphosphate, the natural substrate for viral RNA-directed DNA polymerase. ... d4T-triphosphate appears to compete with thymidine triphosphate for viral RNA-directed DNA polymerase and incorporation into viral DNA. Following incorporation of d4T-triphosphate into the viral DNA chain instead of thymidine triphosphate, synthesis is terminated prematurely because the absence of the 3'-hydroxy group on the drug prevents further 5' to 3' phosphodiester linkages.
This compound is phosphorylated by cellular kinases to the active metabolite this compound triphosphate. This compound triphosphate inhibits the activity of HIV reverse transcriptase both by competing with the natural substrate deoxythymidine triphosphate (Ki =0.0083 to 0.032 uM), and by its incorporation into viral DNA causing a termination of DNA chain elongation because this compound lacks the essential 3'-OH group. This compound triphosphate inhibits cellular DNA polymerase beta and gamma, and markedly reduces the synthesis of mitochondrial DNA.
d4T-Triphosphate can bind to and inhibit some mammalian cellular DNA polymerases, particularly beta- and gamma-polymerases, in vitro, and markedly reduce the synthesis of mitochondrial DNA. ...gamma-polymerase, an enzyme involved in mitochondrial DNA synthesis, is the polymerase most susceptible to inhibition. However, d4T-triphosphate and other dideoxynucleoside triphosphates appear to have much greater affinity for viral RNA-directed DNA polymerase than for mammalian DNA polymerases. ... Inhibition of beta- and gamma-polymerases by these drugs may account, to some extent, for the toxic effects associated with this compound and other nucleosides in humans.
Applications De Recherche Scientifique
Stavudine has several scientific research applications, including:
Chemistry: Used as a model compound to study nucleoside analogs and their chemical properties.
Biology: Employed in research on viral replication and the development of antiviral drugs.
Medicine: Used in clinical studies to evaluate its efficacy and safety in treating HIV infection.
Industry: Utilized in the development of pharmaceutical formulations and drug delivery systems.
Analyse Biochimique
Biochemical Properties
Stavudine is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1) . It is phosphorylated to active metabolites that compete for incorporation into viral DNA . These metabolites inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis .
Cellular Effects
This compound influences cell function by inhibiting the activity of HIV-1 reverse transcriptase (RT), an enzyme crucial for the replication of HIV . By competing with the natural substrate dGTP and by its incorporation into viral DNA, this compound prevents the formation of the 5’ to 3’ phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated .
Molecular Mechanism
This compound exerts its effects at the molecular level by inhibiting the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA . The lack of a 3’-OH group in the incorporated nucleoside analogue prevents the formation of the 5’ to 3’ phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated .
Metabolic Pathways
This compound is involved in the metabolic pathway of HIV replication. It interacts with the enzyme HIV-1 reverse transcriptase (RT) to inhibit the replication of the virus .
Méthodes De Préparation
Voies de synthèse et conditions de réaction : La stavudine peut être synthétisée par différentes méthodes. Une méthode courante consiste à convertir la thymidine en this compound par une série de réactions chimiques. Le processus comprend généralement l'utilisation de réactifs tels que le triphosgène et la pyridine, suivie d'étapes de déprotection pour obtenir le produit final .
Méthodes de production industrielle : Dans les milieux industriels, la this compound est produite à l'aide de techniques de synthèse chimique à grande échelle. Le processus comprend plusieurs étapes, notamment la protection des groupes fonctionnels, des réactions sélectives pour introduire les modifications souhaitées et des étapes de purification pour obtenir de la this compound de haute pureté .
Analyse Des Réactions Chimiques
Types de réactions : La stavudine subit diverses réactions chimiques, notamment :
Oxydation : La this compound peut être oxydée pour former différents métabolites.
Réduction : Les réactions de réduction peuvent modifier les groupes fonctionnels de la this compound.
Substitution : Les réactions de substitution peuvent introduire de nouveaux groupes fonctionnels dans la molécule de this compound.
Réactifs et conditions courants :
Oxydation : Les oxydants courants comprennent le permanganate de potassium et le peroxyde d'hydrogène.
Réduction : Des agents réducteurs tels que le borohydrure de sodium sont utilisés.
Substitution : Des réactifs comme les halogénoalcanes et les nucléophiles sont utilisés.
Principaux produits formés : Les principaux produits formés à partir de ces réactions comprennent divers métabolites et dérivés de this compound modifiés, qui peuvent avoir différentes propriétés pharmacologiques .
4. Applications de la recherche scientifique
La this compound a plusieurs applications de recherche scientifique, notamment :
Chimie : Utilisée comme composé modèle pour étudier les analogues de nucléosides et leurs propriétés chimiques.
Biologie : Utilisée dans la recherche sur la réplication virale et le développement de médicaments antiviraux.
Médecine : Utilisée dans des études cliniques pour évaluer son efficacité et sa sécurité dans le traitement de l'infection par le VIH.
Industrie : Utilisée dans le développement de formulations pharmaceutiques et de systèmes d'administration de médicaments.
5. Mécanisme d'action
La this compound inhibe l'activité de la transcriptase inverse du VIH-1 en entrant en compétition avec le substrat naturel désoxyguanosine triphosphate (dGTP) et en s'incorporant dans l'ADN viral. Cette incorporation entraîne la terminaison de la synthèse de l'ADN, empêchant le virus de se répliquer . La this compound est phosphorylée en métabolites actifs qui entrent en compétition pour l'incorporation dans l'ADN viral, inhibant l'enzyme de manière compétitive .
Comparaison Avec Des Composés Similaires
La stavudine est similaire à d'autres analogues nucléosidiques inhibiteurs de la transcriptase inverse tels que la didanosine et la zalcitabine. Elle présente des propriétés uniques qui la distinguent de ces composés :
Didanosine : également un INTI, mais diffère par sa structure chimique et sa pharmacocinétique.
Zalcitabine : Un autre INTI avec un mécanisme d'action et un profil d'effets secondaires différents.
Liste des composés similaires :
- Didanosine
- Zalcitabine
- Zidovudine
La structure chimique et le mécanisme d'action uniques de la this compound en font un composé précieux dans le traitement de l'infection par le VIH et un sujet de recherche scientifique continue.
Propriétés
IUPAC Name |
1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XNKLLVCARDGLGL-JGVFFNPUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=CN(C(=O)NC1=O)C2C=CC(O2)CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC1=CN(C(=O)NC1=O)[C@H]2C=C[C@H](O2)CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H12N2O4 | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID1023819 | |
| Record name | Stavudine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1023819 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
224.21 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
2',3'-didehydro-3'-deoxythymidine appears as white crystalline solid or powder. Odorless. (NTP, 1992), Solid | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
50 to 100 mg/mL at 70 °F (NTP, 1992), 5-10 g/100 mL at 21 °C, 30 mg/mL in propylene glycol at 23 °C, In water, 83 mg/mL at 23 °C, 4.05e+01 g/L | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
9.5X10-12 mm Hg at 25 °C /Estimated/ | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Stavudine inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA., Enzymatic conversion of stavudine to d4T-triphosphate appears to be complex, involving several steps and enzymes. Stavudine is first converted to dideoxydidehydrothymidine-5'-monophosphate (d4T-monophosphate) by thymidine kinase. Subsequently, d4T-monophosphate is converted to dideoxydidehydrothymidine-5'-diphosphate (d4T-diphosphate), and then to d4T-triphosphate, presumably by the same cellular kinases involved in the metabolism of zidovudine. ... d4T-Triphosphate is a structural analog of thymidine triphosphate, the natural substrate for viral RNA-directed DNA polymerase. ... d4T-triphosphate appears to compete with thymidine triphosphate for viral RNA-directed DNA polymerase and incorporation into viral DNA. Following incorporation of d4T-triphosphate into the viral DNA chain instead of thymidine triphosphate, synthesis is terminated prematurely because the absence of the 3'-hydroxy group on the drug prevents further 5' to 3' phosphodiester linkages., Stavudine is phosphorylated by cellular kinases to the active metabolite stavudine triphosphate. Stavudine triphosphate inhibits the activity of HIV reverse transcriptase both by competing with the natural substrate deoxythymidine triphosphate (Ki =0.0083 to 0.032 uM), and by its incorporation into viral DNA causing a termination of DNA chain elongation because stavudine lacks the essential 3'-OH group. Stavudine triphosphate inhibits cellular DNA polymerase beta and gamma, and markedly reduces the synthesis of mitochondrial DNA., d4T-Triphosphate can bind to and inhibit some mammalian cellular DNA polymerases, particularly beta- and gamma-polymerases, in vitro, and markedly reduce the synthesis of mitochondrial DNA. ...gamma-polymerase, an enzyme involved in mitochondrial DNA synthesis, is the polymerase most susceptible to inhibition. However, d4T-triphosphate and other dideoxynucleoside triphosphates appear to have much greater affinity for viral RNA-directed DNA polymerase than for mammalian DNA polymerases. ... Inhibition of beta- and gamma-polymerases by these drugs may account, to some extent, for the toxic effects associated with stavudine and other nucleosides in humans. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to off white crystalline solid, Colorless granular solid from ethanol/benzene | |
CAS No. |
3056-17-5 | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=3056-17-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Stavudine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0003056175 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | stavudine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759897 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Stavudine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1023819 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1-((2R, 5S)-5-(hydroxymethyl)-2,5-dihydro-2-furanyl)-5-methyl-2,4(1H, 3H)-pyrimidinedione | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | STAVUDINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/BO9LE4QFZF | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
318 to 320 °F (NTP, 1992), 159-160 °C, 165-166 °C, 159 - 160 °C | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.


![3-[(4S)-5-Oxo-2-(trifluoromethyl)-1,4,5,6,7,8-hexahydroquinolin-4-YL]benzonitrile](/img/structure/B1682409.png)
![3-cyano-N-[(2S)-2-(3,4-dichlorophenyl)-4-[4-[2-[(S)-methylsulfinyl]phenyl]piperidin-1-yl]butyl]-N-methylnaphthalene-1-carboxamide](/img/structure/B1682412.png)