Perampanel
Vue d'ensemble
Description
Le perampanel est un nouveau médicament antiépileptique qui agit comme un antagoniste sélectif et non compétitif du récepteur de l'acide α-amino-3-hydroxy-5-méthyl-4-isoxazolepropionique. Il est principalement utilisé comme traitement d'appoint pour le traitement des crises partielles et des crises tonico-cloniques généralisées primaires chez les patients atteints d'épilepsie . Le this compound est commercialisé sous le nom de marque Fycompa et a été approuvé dans plus de 35 pays .
Mécanisme D'action
Target of Action
Perampanel is a non-competitive antagonist of the AMPA glutamate receptor . Glutamate is the primary neurotransmitter regulating excitatory synaptic transmission in the brain . The AMPA receptor is one of the ligand-gated ion channels for glutamate .
Mode of Action
It is specifically engineered to block glutamate activity at postsynaptic AMPA receptors .
Biochemical Pathways
This compound affects the up-stream regulatory pathways of GluA1 phosphorylation including protein kinase C (PKC), Ca2±calmodulin-dependent protein kinase II (CAMKII), protein kinase A (PKA), extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and protein phosphatase (PP) 1, PP2A, and PP2B . These proteins form receptor signaling complexes with AMPARs and can modulate their trafficking, opening, closing, desensitization, pharmacological properties, and permeability, providing fine-tuning of the AMPA receptor functions in space and time .
Pharmacokinetics
This compound is rapidly absorbed with Tmax values of 0.5–2.5 h and with a bioavailability of 100%. The volume of distribution is 1.1 L/kg and protein binding is 95% . This compound is highly metabolized by CYP3A4 and/or CYP3A5 primary oxidation and by sequential glucuronidation . This compound is eliminated mostly in the feces (48%) and to a lesser extent in the urine (22%) .
Result of Action
This compound has been shown to have antiproliferative effects on glioblastoma cell lines . It also significantly attenuated oxygen glucose deprivation (OGD)-induced loss of cell viability, release of lactate dehydrogenase, and apoptotic cell death in a dose-dependent manner . Furthermore, this compound has been reported to alleviate early brain injury following subarachnoid hemorrhage and traumatic brain injury by reducing reactive oxygen species, apoptosis, autophagy, and necroptosis .
Action Environment
Environmental factors such as iron overload can influence the action of this compound. Iron overload in the neonatal period induces persistent memory deficits and increases oxidative stress and apoptotic markers. The neuronal insult caused by iron excess generates an energetic imbalance that can alter glutamate concentrations and thus trigger excitotoxicity. This compound, as a reversible AMPA receptor antagonist, has been shown to improve memory in rodents subjected to iron overload in the neonatal period .
Analyse Biochimique
Biochemical Properties
Perampanel plays a crucial role in inhibiting neuronal excitation in the central nervous system by blocking AMPA receptors. These receptors are ligand-gated ion channels that mediate fast synaptic transmission in the central nervous system. By inhibiting these receptors, this compound reduces the excitatory neurotransmission that can lead to seizures . This compound interacts with various biomolecules, including proteins such as protein kinase C (PKC), Ca2±calmodulin-dependent protein kinase II (CAMKII), protein kinase A (PKA), extracellular signal-regulated kinase 1/2 (ERK1/2), and c-Jun N-terminal kinase (JNK) .
Cellular Effects
This compound affects various types of cells and cellular processes. It influences cell function by modulating cell signaling pathways, gene expression, and cellular metabolism. In normal and epileptic rats, this compound has been shown to affect the phosphorylation of GluA1, a subunit of the AMPA receptor, through the regulation of multiple molecules such as CAMKII, PKA, JNK, and protein phosphatase 2B (PP2B) . Additionally, this compound has been found to exert protective effects against traumatic brain injury by reducing neuronal apoptosis and inhibiting lipid peroxidation .
Molecular Mechanism
The molecular mechanism of this compound involves its action as a non-competitive antagonist of the AMPA receptor. By binding to a site distinct from the glutamate binding site, this compound inhibits the receptor’s activity, leading to decreased neuronal excitation . This inhibition is achieved through negative allosteric modulation, which prevents the receptor from undergoing the conformational changes necessary for ion channel opening . This compound’s effects on gene expression include the modulation of signaling pathways that regulate the phosphorylation of AMPA receptor subunits .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound have been observed to change over time. This compound is stable and maintains its efficacy in reducing seizure activity over extended periods. In studies involving epileptic rats, this compound effectively inhibited spontaneous seizure activities and maintained its neuroprotective effects without negatively impacting cognitive abilities . Additionally, this compound has been shown to resolve status epilepticus within 24 hours in patients with inflammatory or autoimmune etiologies .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. In a rat model of chronic epilepsy, fully adherent rats demonstrated a significant reduction in seizure frequency with this compound treatment, while nonadherent rats experienced an increase in seizure frequency . High doses of this compound have been associated with adverse effects such as vomiting and decreased activity in beagle dogs . The therapeutic index of this compound is narrow, and optimal dosing strategies are essential to maximize its efficacy while minimizing adverse effects .
Metabolic Pathways
This compound is primarily metabolized by the cytochrome P450 enzymes CYP3A4 and CYP3A5 through oxidative metabolism and sequential glucuronidation . The drug is highly metabolized in the liver, and its metabolites are not pharmacologically active . The metabolic pathways of this compound involve interactions with enzymes that regulate its biotransformation and elimination .
Transport and Distribution
This compound is rapidly absorbed after oral ingestion, with a bioavailability of 100% and a volume of distribution of 1.1 L/kg . It is extensively bound to plasma proteins, primarily α1-acid glycoprotein and albumin . This compound undergoes extensive metabolism in the liver, and less than 0.12% of the administered dose is excreted unchanged in the urine . The drug is widely distributed throughout tissues, and its pharmacokinetics are influenced by the presence of enzyme-inducing antiseizure medications .
Subcellular Localization
The subcellular localization of this compound is primarily associated with its target, the AMPA receptor, which is located on the postsynaptic membranes of neurons . This compound’s activity is influenced by its ability to bind to the AMPA receptor and inhibit its function. The drug’s localization to specific subcellular compartments is essential for its therapeutic effects in reducing neuronal excitation and preventing seizures .
Méthodes De Préparation
La synthèse du perampanel implique plusieurs étapes, commençant par la protection du groupe hydroxyle à l'aide d'un groupe protecteur résistant aux alcalis tel que le dihydropyranyle ou le trityle . L'intermédiaire est ensuite soumis à diverses conditions réactionnelles, notamment l'utilisation d'acide p-toluènesulfonique monohydraté et de dihydropyrane, pour obtenir le produit final . Les méthodes de production industrielle impliquent généralement la chromatographie liquide haute performance pour la quantification et la validation du this compound dans les échantillons de plasma .
Analyse Des Réactions Chimiques
Le perampanel subit plusieurs types de réactions chimiques, notamment l'oxydation, la réduction et la substitution. Les réactifs couramment utilisés dans ces réactions comprennent l'acétonitrile, le méthanol et l'acide orthophosphorique . Les principaux produits formés à partir de ces réactions sont généralement analysés par chromatographie liquide haute performance et détection par fluorescence .
Applications de recherche scientifique
Le this compound a un large éventail d'applications de recherche scientifique, en particulier dans les domaines de la chimie, de la biologie, de la médecine et de l'industrie. En médecine, il est utilisé comme médicament antiépileptique pour le traitement des crises partielles réfractaires et des crises tonico-cloniques généralisées primaires . En recherche biologique, le this compound est étudié pour ses propriétés anti-inflammatoires et neuroprotectrices, en particulier dans le traitement du statut épileptique avec une étiologie inflammatoire suspectée . De plus, le this compound est utilisé dans la recherche pharmacologique pour étudier ses effets sur le récepteur de l'acide α-amino-3-hydroxy-5-méthyl-4-isoxazolepropionique .
Mécanisme d'action
Le this compound exerce ses effets en inhibant sélectivement le récepteur de l'acide α-amino-3-hydroxy-5-méthyl-4-isoxazolepropionique, qui est impliqué dans la transmission synaptique excitatrice dans le cerveau . En bloquant l'activité de ce récepteur, le this compound réduit l'excitation neuronale et aide à contrôler les crises . Les cibles moléculaires et les voies exactes impliquées dans ce mécanisme sont encore à l'étude, mais il est connu que le this compound a une demi-vie terminale prolongée et est fortement lié aux protéines plasmatiques .
Applications De Recherche Scientifique
Perampanel has a wide range of scientific research applications, particularly in the fields of chemistry, biology, medicine, and industry. In medicine, it is used as an antiepileptic drug for the treatment of refractory partial-onset seizures and primary generalized tonic-clonic seizures . In biological research, this compound is studied for its anti-inflammatory and neuroprotective properties, particularly in the treatment of status epilepticus with suspected inflammatory etiology . Additionally, this compound is used in pharmacological research to study its effects on the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor .
Comparaison Avec Des Composés Similaires
Le perampanel est unique parmi les médicaments antiépileptiques en raison de son inhibition sélective du récepteur de l'acide α-amino-3-hydroxy-5-méthyl-4-isoxazolepropionique . Des composés similaires comprennent d'autres médicaments antiépileptiques qui ciblent des récepteurs différents, tels que la lévétiracétam et la lamotrigine . Contrairement à ces médicaments, le this compound offre la commodité d'une administration une fois par jour et a démontré qu'il offrait un contrôle durable des crises chez les patients atteints d'épilepsie pharmaco-résistante .
Propriétés
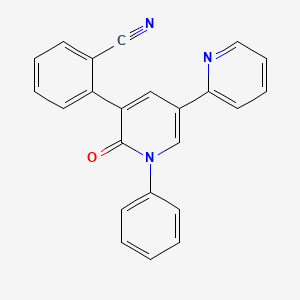
IUPAC Name |
2-(2-oxo-1-phenyl-5-pyridin-2-ylpyridin-3-yl)benzonitrile | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H15N3O/c24-15-17-8-4-5-11-20(17)21-14-18(22-12-6-7-13-25-22)16-26(23(21)27)19-9-2-1-3-10-19/h1-14,16H | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PRMWGUBFXWROHD-UHFFFAOYSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CC=C(C=C1)N2C=C(C=C(C2=O)C3=CC=CC=C3C#N)C4=CC=CC=N4 | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H15N3O | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID80191501 | |
| Record name | Perampanel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80191501 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
349.4 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Practically insoluble in water. | |
| Details | From FDA label. | |
| Record name | Perampanel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08883 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
The exact mechanism of action of perampanel in seizures is not yet determined, but it is known that perampanel decreases neuronal excitation by non-competitive ihibition of the AMPA receptor. | |
| Record name | Perampanel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08883 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
380917-97-5 | |
| Record name | Perampanel | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=380917-97-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Perampanel [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0380917975 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Perampanel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08883 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Perampanel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80191501 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile hydrate(4:3) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PERAMPANEL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/H821664NPK | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details










Synthesis routes and methods II
Procedure details







Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.
![L-Valine, N-[N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanyl]-](/img/structure/B3395813.png)
![4-nitro-7-oxidothieno[2,3-b]pyridin-7-ium](/img/structure/B3395836.png)
![4-chloro-7-oxidothieno[2,3-b]pyridin-7-ium](/img/structure/B3395841.png)