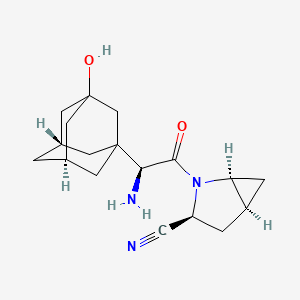
Saxagliptin
Vue d'ensemble
Description
Saxagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor which is used in combination with diet and exercise in the therapy of type 2 diabetes, either alone or in combination with other oral hypoglycemic agents. This compound is a relatively new medication and has yet to be implicated in causing clinically apparent liver injury.
This compound is a potent, selective and competitive, cyanopyrrolidine-based, orally bioavailable inhibitor of dipeptidyl peptidase 4 (DPP-4), with hypoglycemic activity. This compound is metabolized into an, although less potent, active mono-hydroxy metabolite.
Mécanisme D'action
Type 2 diabetes (T2D) is one of the major risk factors associated with Alzheimer's disease (AD). Recent studies have found similarities in molecular mechanisms that underlie the respective degenerative developments in the two diseases. Pharmacological agents, such as dipeptidyl peptidase-4 (DPP-4) inhibitors, which increase the level of glucagon-like peptide-1 (GLP-1) and ameliorate T2D, have become valuable candidates as disease modifying agents in the treatment of AD. In addition, endogenous GLP-1 levels decrease amyloid beta (Abeta) peptide and tau phosphorylation in AD. The present study examines the efficacy of Saxagliptin, a DPP-4 inhibitor in a streptozotocin (STZ) induced rat model of AD. Three months following induction of AD by intracerebral administration of streptozotocin, animals were orally administered this compound (0.25, 0.5 and 1 mg/kg) for 60 days. The effect of the DPP-4 inhibitor on hippocampal GLP-1 levels, Abeta burden, tau phosphorylation, inflammatory markers and memory retention were evaluated. The results reveal an attenuation of Abeta, tau phosphorylation and inflammatory markers and an improvement in hippocampal GLP-1 and memory retention following treatment. This remarkable therapeutic effect of this compound mediated through DPP-4 inhibition demonstrates a unique mechanism for Abeta and tau clearance by increasing GLP-1 levels and reverses the behavioural deficits and pathology observed in AD.
This compound inhibits dipeptidyl peptidase-4 (DPP-4), an enzyme that inactivates incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Both this compound and its active metabolite (5-hydroxy this compound) are more selective for inhibition of DPP-4 than for DPP-8 or DPP-9. This compound increases circulating levels of GLP-1 and GIP in a glucose-dependent manner. GLP-1 and GIP stimulate insulin secretion from pancreatic beta-cells in a glucose-dependent manner (i.e., when glucose concentrations are normal or elevated). GLP-1 also decreases glucagon secretion from pancreatic alpha-cells, leading to reduced hepatic glucose production. This compound lowers fasting plasma glucose concentrations and reduces glucose excursions following a glucose load or meal in patients with type 2 diabetes mellitus.
Propriétés
IUPAC Name |
(1S,3S,5S)-2-[(2S)-2-amino-2-[(5S,7R)-3-hydroxy-1-adamantyl]acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10-,11+,12-,13+,14+,15-,17?,18?/m1/s1 | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
QGJUIPDUBHWZPV-KFHUQKARSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1C2CC2N(C1C#N)C(=O)C(C34CC5CC(C3)CC(C5)(C4)O)N | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1[C@@H]2C[C@@H]2N([C@@H]1C#N)C(=O)[C@H](C34C[C@H]5C[C@@H](C3)CC(C5)(C4)O)N | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H25N3O2 | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
315.4 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Type 2 diabetes (T2D) is one of the major risk factors associated with Alzheimer's disease (AD). Recent studies have found similarities in molecular mechanisms that underlie the respective degenerative developments in the two diseases. Pharmacological agents, such as dipeptidyl peptidase-4 (DPP-4) inhibitors, which increase the level of glucagon-like peptide-1 (GLP-1) and ameliorate T2D, have become valuable candidates as disease modifying agents in the treatment of AD. In addition, endogenous GLP-1 levels decrease amyloid beta (Abeta) peptide and tau phosphorylation in AD. The present study examines the efficacy of Saxagliptin, a DPP-4 inhibitor in a streptozotocin (STZ) induced rat model of AD. Three months following induction of AD by intracerebral administration of streptozotocin, animals were orally administered Saxagliptin (0.25, 0.5 and 1 mg/kg) for 60 days. The effect of the DPP-4 inhibitor on hippocampal GLP-1 levels, Abeta burden, tau phosphorylation, inflammatory markers and memory retention were evaluated. The results reveal an attenuation of Abeta, tau phosphorylation and inflammatory markers and an improvement in hippocampal GLP-1 and memory retention following treatment. This remarkable therapeutic effect of Saxagliptin mediated through DPP-4 inhibition demonstrates a unique mechanism for Abeta and tau clearance by increasing GLP-1 levels and reverses the behavioural deficits and pathology observed in AD., Saxagliptin inhibits dipeptidyl peptidase-4 (DPP-4), an enzyme that inactivates incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Both saxagliptin and its active metabolite (5-hydroxy saxagliptin) are more selective for inhibition of DPP-4 than for DPP-8 or DPP-9. Saxagliptin increases circulating levels of GLP-1 and GIP in a glucose-dependent manner. GLP-1 and GIP stimulate insulin secretion from pancreatic beta-cells in a glucose-dependent manner (i.e., when glucose concentrations are normal or elevated). GLP-1 also decreases glucagon secretion from pancreatic alpha-cells, leading to reduced hepatic glucose production. Saxagliptin lowers fasting plasma glucose concentrations and reduces glucose excursions following a glucose load or meal in patients with type 2 diabetes mellitus. | |
| Details | American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 3209 | |
| Record name | Saxagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8199 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
361442-04-8 | |
| Record name | Saxagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8199 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.


![3-[4-[5-fluoro-2-(trifluoromethyl)phenyl]pyridin-2-yl]-2H-1,2,4-oxadiazol-5-one](/img/structure/B8054643.png)
![3-[4-[3-fluoro-2-(trifluoromethyl)phenyl]pyridin-2-yl]-2H-1,2,4-oxadiazol-5-one](/img/structure/B8054647.png)
![3-[4-[2,5-bis(trifluoromethyl)phenyl]pyridin-2-yl]-2H-1,2,4-oxadiazol-5-one](/img/structure/B8054650.png)
![3-[4-(2-fluorophenyl)pyridin-2-yl]-2H-1,2,4-oxadiazol-5-one](/img/structure/B8054656.png)
![3-[4-(2-methylphenyl)pyridin-2-yl]-2H-1,2,4-oxadiazol-5-one](/img/structure/B8054658.png)