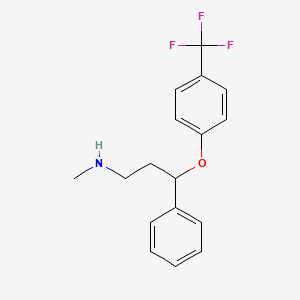
フルオキセチン
概要
説明
フルオキセチンは、選択的セロトニン再取り込み阻害薬(SSRI)のクラスに属する広く使用されている抗うつ薬です。 主に、主要なうつ病、強迫性障害、神経性過食症、パニック障害、月経前不快気分障害の治療に処方されます 。フルオキセチンは1980年代後半に初めて導入され、以来、世界中で最も一般的に処方される抗うつ薬の1つになっています。
2. 製法
合成経路と反応条件: フルオキセチンは、複数の合成経路を通じて合成することができます。一般的な方法の1つは、塩基の存在下で3-クロロプロピオフェノンを4-(トリフルオロメチル)フェノールと反応させて3-(4-(トリフルオロメチル)フェノキシ)プロピオフェノンを生成することです。 この中間体を次にメチルアミンと反応させてフルオキセチンを生成します 。
工業生産方法: フルオキセチンの工業生産では、多くの場合、連続フロー処理技術が採用されます。 この方法は、従来のバッチ処理と比較して、反応制御の改善、収率の向上、安全性の強化などの利点があります 。
科学的研究の応用
Fluoxetine has a wide range of scientific research applications:
Chemistry: Fluoxetine is used as a reference compound in the development of new selective serotonin reuptake inhibitors.
Biology: It is employed in studies investigating the role of serotonin in various biological processes.
作用機序
フルオキセチンは、脳内の神経伝達物質であるセロトニンの再取り込みを阻害することで効果を発揮します。 この阻害により、シナプス間隙のセロトニンレベルが上昇し、神経伝達が増強され、気分が改善されます 。 フルオキセチンは主にセロトニントランスポーターを標的とする一方、ノルエピネフリンおよびドーパミントランスポーターに対してもわずかな効果があります 。
生化学分析
Biochemical Properties
Fluoxetine plays a crucial role in biochemical reactions by inhibiting the reuptake of serotonin, a neurotransmitter that regulates mood, appetite, and sleep . This inhibition is achieved by binding to the serotonin transporter (SERT) on the presynaptic terminal, preventing the reabsorption of serotonin into the presynaptic neuron . Fluoxetine interacts with various enzymes and proteins, including cytochrome P450 enzymes such as CYP2D6, CYP2C19, CYP2C9, CYP3A4, and CYP3A5, which are involved in its metabolism . These interactions result in the formation of its active metabolite, norfluoxetine .
Cellular Effects
Fluoxetine has significant effects on various types of cells and cellular processes. It influences cell function by increasing the levels of serotonin in the synaptic cleft, which enhances serotonergic neurotransmission . This increase in serotonin levels affects cell signaling pathways, gene expression, and cellular metabolism. Fluoxetine has been shown to impact neurogenesis, brain-derived neurotrophic factor levels, and hypothalamic-pituitary-adrenal axis activity . Additionally, it can alter the activity of other neurotransmitter systems, such as dopamine and norepinephrine, by indirectly modulating their signaling pathways .
Molecular Mechanism
The molecular mechanism of fluoxetine involves its action as a selective serotonin reuptake inhibitor. Fluoxetine binds to the serotonin transporter (SERT) at a site other than the serotonin binding site, allosterically inhibiting the transporter . This inhibition prevents the reuptake of serotonin into the presynaptic neuron, leading to increased levels of serotonin in the synaptic cleft . Fluoxetine also interacts with cytochrome P450 enzymes, particularly CYP2D6, which metabolizes fluoxetine into its active metabolite, norfluoxetine . This metabolite further contributes to the inhibition of serotonin reuptake and prolongs the drug’s effects .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of fluoxetine change over time. Fluoxetine is well absorbed after oral administration and has a long plasma half-life, ranging from 1 to 4 days for fluoxetine and 7 to 15 days for its metabolite, norfluoxetine . Chronic administration of fluoxetine leads to sustained increases in serotonin levels and alterations in receptor sensitivity . Studies have shown that fluoxetine can cause internalization and degradation of the serotonin transporter (SERT) after chronic treatment . Additionally, the stability and degradation of fluoxetine and its metabolites can influence their long-term effects on cellular function .
Dosage Effects in Animal Models
The effects of fluoxetine vary with different dosages in animal models. Chronic administration of fluoxetine at doses of 10 to 18 mg/kg/day has been shown to reduce anxiety-related behaviors and increase swimming behavior in the forced swim test . Higher doses of fluoxetine can lead to adverse effects, such as increased anxiety and reduced locomotion . These dosage-dependent effects highlight the importance of optimizing fluoxetine dosage to achieve therapeutic benefits while minimizing adverse effects.
Metabolic Pathways
Fluoxetine is extensively metabolized in the liver by cytochrome P450 enzymes, particularly CYP2D6, CYP2C19, CYP2C9, CYP3A4, and CYP3A5 . The primary metabolic pathway involves the N-demethylation of fluoxetine to form its active metabolite, norfluoxetine . This metabolite retains the ability to inhibit serotonin reuptake and contributes to the overall pharmacological effects of fluoxetine . The involvement of multiple cytochrome P450 enzymes in fluoxetine metabolism can lead to variations in metabolic flux and metabolite levels, depending on individual genetic polymorphisms .
Transport and Distribution
Fluoxetine is highly lipophilic and has a large volume of distribution, allowing it to accumulate in various tissues . It is extensively bound to plasma proteins, with a protein binding rate of approximately 95% . Fluoxetine is transported across cell membranes and distributed within cells and tissues, where it exerts its pharmacological effects . The drug’s distribution is influenced by its interactions with transporters and binding proteins, which can affect its localization and accumulation in specific compartments .
Subcellular Localization
Fluoxetine’s subcellular localization is primarily associated with the serotonin transporter (SERT) on the plasma membrane of presynaptic neurons . Chronic administration of fluoxetine leads to the internalization and degradation of SERT, reducing its availability on the cell surface . This internalization process is accompanied by changes in the subcellular localization of other proteins, such as 5-HT1A receptors, which are involved in the regulation of serotonin signaling . The targeting signals and post-translational modifications of fluoxetine and its metabolites play a crucial role in directing them to specific compartments or organelles within the cell .
準備方法
Synthetic Routes and Reaction Conditions: Fluoxetine can be synthesized through multiple synthetic routes. One common method involves the reaction of 3-chloropropiophenone with 4-(trifluoromethyl)phenol in the presence of a base to form 3-(4-(trifluoromethyl)phenoxy)propiophenone. This intermediate is then reacted with methylamine to produce fluoxetine .
Industrial Production Methods: Industrial production of fluoxetine often employs continuous flow processing techniques. This method offers advantages over traditional batch processing, including improved reaction control, higher yields, and enhanced safety .
化学反応の分析
反応の種類: フルオキセチンは、以下を含むさまざまな化学反応を起こします。
酸化: フルオキセチンは、酸化されて主要な活性代謝物であるノルフルオキセチンを生成することができます。
還元: 還元反応はそれほど一般的ではありませんが、特定の条件下では起こり得ます。
置換: フルオキセチンは、特に強い求核剤の存在下で置換反応を起こすことができます。
一般的な試薬と条件:
酸化: 一般的な酸化剤には、過マンガン酸カリウムと過酸化水素があります。
還元: 水素化リチウムアルミニウムなどの還元剤を使用できます。
置換: 水素化ナトリウムなどの強い求核剤がしばしば使用されます。
主な生成物:
酸化: ノルフルオキセチン
還元: 還元されたフルオキセチン誘導体
置換: 置換されたフルオキセチン化合物
4. 科学研究への応用
フルオキセチンは、科学研究において幅広い応用範囲があります。
化学: フルオキセチンは、新しい選択的セロトニン再取り込み阻害薬の開発における参照化合物として使用されます。
生物学: さまざまな生物学的プロセスにおけるセロトニンの役割を調査する研究で使用されます。
類似化合物との比較
フルオキセチンは、しばしば、以下のような他の選択的セロトニン再取り込み阻害薬と比較されます。
- シタロプラム
- エシタロプラム
- パロキセチン
- セルタリン
独自性: フルオキセチンは、長い半減期が特徴であり、1日1回の投与が可能になり、離脱症状のリスクが軽減されます 。 さらに、その活性代謝物であるノルフルオキセチンが、その持続的な治療効果に貢献しています 。
特性
IUPAC Name |
N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RTHCYVBBDHJXIQ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CNCCC(C1=CC=CC=C1)OC2=CC=C(C=C2)C(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H18F3NO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
59333-67-4 (hydrochloride) | |
| Record name | Fluoxetine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0054910893 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID7023067 | |
| Record name | Fluoxetine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023067 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
309.33 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Fluoxetine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014615 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
395.1°C at 760 mmHg | |
| Record name | Fluoxetine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00472 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Solubility |
insoluble, 1.70e-03 g/L | |
| Record name | Fluoxetine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00472 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fluoxetine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014615 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The monoaminergic hypothesis of depression emerged in 1965 and linked depression with dysfunction of neurotransmitters such as noradrenaline and serotonin. Indeed, low levels of serotonin have been observed in the cerebrospinal fluid of patients diagnosed with depression. As a result of this hypothesis, drugs that modulate levels of serotonin such as fluoxetine were developed. Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) and as the name suggests, it exerts it's therapeutic effect by inhibiting the presynaptic reuptake of the neurotransmitter serotonin. As a result, levels of 5-hydroxytryptamine (5-HT) are increased in various parts of the brain. Further, fluoxetine has high affinity for 5-HT transporters, weak affinity for noradrenaline transporters and no affinity for dopamine transporters indicating that it is 5-HT selective. Fluoxetine interacts to a degree with the 5-HT2C receptor and it has been suggested that through this mechanism, it is able to increase noradrenaline and dopamine levels in the prefrontal cortex. | |
| Record name | Fluoxetine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00472 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
54910-89-3, 57226-07-0 | |
| Record name | Fluoxetine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=54910-89-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Fluoxetine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0054910893 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Fluoxetine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00472 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | NSC-283480 | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=283480 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Fluoxetine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023067 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Benzenepropanamine, N-methyl-γ-[4-(trifluoromethyl)phenoxy] | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.125.370 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | FLUOXETINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/01K63SUP8D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Fluoxetine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014615 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
179 - 182 °C | |
| Record name | Fluoxetine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00472 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fluoxetine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014615 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details




















Synthesis routes and methods III
Procedure details








Synthesis routes and methods IV
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Fluoxetine?
A1: Fluoxetine primarily acts as a selective serotonin reuptake inhibitor (SSRI). It achieves this by binding to the serotonin transporter (SERT) and blocking the reuptake of serotonin from the synaptic cleft. This leads to an increase in extracellular serotonin levels, enhancing serotonergic neurotransmission [, , ].
Q2: Are there other mechanisms through which Fluoxetine exerts its effects, beyond SERT inhibition?
A2: While SERT inhibition is the primary mechanism, research suggests Fluoxetine might also directly interact with certain serotonin receptors. For instance, studies in C. elegans revealed that Fluoxetine binds to the SER-7 receptor, influencing downstream signaling pathways involved in acetylcholine, GABA, and glutamate neurotransmission. This suggests a potential SERT-independent mechanism of action [].
Q3: How does Fluoxetine impact neuronal plasticity?
A3: Studies show that Fluoxetine can influence neuroplasticity, particularly neurogenesis, in various brain regions. In rodents, chronic Fluoxetine treatment was found to increase cell proliferation in the hippocampus, frontal cortex, cerebellum, and amygdala []. It also stimulated the expression of brain-derived neurotrophic factor (BDNF) in the hippocampus, a neurotrophin crucial for neuronal survival and growth [, , ].
Q4: What is the molecular formula and weight of Fluoxetine?
A4: Due to the scope of this scientific deep dive focusing on the provided research papers, specific structural details like molecular formula and weight are not included. This information can be readily found in publicly available chemical databases like PubChem or DrugBank.
Q5: Have computational methods been used to study Fluoxetine and its interactions?
A8: Yes, computational modeling, particularly physiologically based pharmacokinetic (PBPK) modeling, has been employed to predict and understand Fluoxetine and its metabolite norfluoxetine's pharmacokinetic profiles []. These models incorporate physiological parameters and drug-specific properties to simulate drug behavior in the body, aiding in dosage optimization and prediction of drug interactions.
Q6: How do structural modifications of Fluoxetine affect its activity and selectivity?
A9: While the provided research doesn't delve into specific structural modifications of Fluoxetine, it highlights the importance of chirality. Fluoxetine and its metabolite norfluoxetine exist as enantiomers (mirror image molecules). Research shows that both (R)- and (S)-enantiomers of Fluoxetine and norfluoxetine act as potent inhibitors of the cytochrome P450 2D6 enzyme, which is involved in drug metabolism []. Interestingly, the (S)-enantiomers exhibit higher potency for inhibition compared to their (R)- counterparts, suggesting stereoselective interactions with the enzyme [, ].
Q7: How is Fluoxetine metabolized in the body?
A12: Fluoxetine undergoes extensive metabolism in the liver, primarily by cytochrome P450 enzymes, particularly CYP2D6, CYP2C19, and CYP3A4 [, ]. N-demethylation is the major metabolic pathway, resulting in the formation of its active metabolite, norfluoxetine [, , ].
Q8: Does the metabolism of Fluoxetine differ between adults and fetuses?
A13: Yes, significant differences exist in Fluoxetine metabolism between adults and fetuses. Studies in humans and sheep demonstrate that while Fluoxetine readily crosses the placenta, fetal metabolic capacity is limited compared to adults []. This results in lower or even undetectable levels of norfluoxetine in fetal circulation [].
Q9: Are there any age-related differences in Fluoxetine pharmacokinetics?
A14: Research indicates age-related differences, particularly in elderly populations. Studies show that elderly patients, especially women, tend to have higher plasma concentrations of Fluoxetine and norfluoxetine compared to younger adults []. Additionally, the terminal half-life of norfluoxetine is prolonged in individuals over 75 years old, with elderly women exhibiting a slower elimination rate than men of the same age group [].
Q10: How does Fluoxetine's pharmacokinetic profile contribute to its long duration of action?
A15: Fluoxetine's long duration of action can be attributed to its slow elimination rate and the presence of its active metabolite, norfluoxetine. Both Fluoxetine and norfluoxetine have relatively long half-lives, meaning they remain in the body for an extended period. This sustained presence contributes to their therapeutic effects but also necessitates careful dosage adjustments, especially in populations with altered drug metabolism, such as the elderly [, ].
Q11: What in vitro models have been used to study Fluoxetine's effects?
A16: In vitro studies have utilized various models to investigate Fluoxetine's effects. For instance, researchers have used cultured rat astrocytes to study the impact of Fluoxetine on glial cell line-derived neurotrophic factor (GDNF) synthesis and release. These studies showed that Fluoxetine stimulates GDNF release from astrocytes, suggesting a potential mechanism for its neuroprotective properties [].
Q12: What animal models have been employed to investigate Fluoxetine's effects on depression and other conditions?
A17: Rodent models, particularly rats, have been widely used to study the effects of Fluoxetine. For example, the chronic unpredictable mild stress (CUMS) model in rats has been employed to mimic the behavioral and physiological aspects of depression. Studies using this model showed that CUMS leads to behavioral changes like decreased sucrose preference and increased immobility in the forced swim test, indicative of depressive-like behavior [, ]. Administration of Fluoxetine in these models was found to reverse some of these behavioral deficits, suggesting antidepressant-like effects [, ].
Q13: Has Fluoxetine been investigated for its potential in conditions beyond depression?
A18: Yes, research has explored Fluoxetine's therapeutic potential in various conditions beyond depression. Studies have investigated its efficacy in treating conditions like obsessive-compulsive disorder (OCD), post-traumatic stress disorder (PTSD), binge eating disorder (BED), and acne excoriée [, , , ]. These studies have provided valuable insights into Fluoxetine's potential broader therapeutic applications.
Q14: What do clinical trials reveal about Fluoxetine's efficacy in treating major depressive disorder?
A19: Numerous clinical trials have established Fluoxetine's efficacy in treating major depressive disorder. Double-blind, placebo-controlled trials have demonstrated significant improvements in depressive symptoms, as measured by standardized rating scales like the Hamilton Rating Scale for Depression (HAMD), in patients receiving Fluoxetine compared to those receiving placebo [, , ].
Q15: Has the efficacy of Fluoxetine been compared to other antidepressants in clinical trials?
A20: Yes, several clinical trials have compared Fluoxetine's efficacy to other antidepressant medications. For instance, double-blind studies have compared Fluoxetine to bupropion, finding similar efficacy in relieving depression and anxiety symptoms []. Other trials have compared Fluoxetine to tricyclic antidepressants, with varying results depending on the specific tricyclic used and patient populations studied [].
Q16: What are some potential reasons for the observed variability in response to Fluoxetine treatment?
A21: The variability in individual responses to Fluoxetine can be attributed to various factors. Genetic variations in drug-metabolizing enzymes, particularly CYP2D6, can significantly influence Fluoxetine's pharmacokinetics and consequently, its clinical efficacy [, ]. Polymorphisms in genes encoding serotonin receptors and transporters might also contribute to differences in treatment response [, ].
Q17: What potential biomarkers have been explored to predict Fluoxetine efficacy or monitor treatment response?
A25: One potential biomarker that has been investigated is the P300 component of event-related potentials (ERPs), a neurophysiological measure of cognitive function. Studies have explored the relationship between P300 parameters, such as latency and amplitude, and treatment response to antidepressants like Fluoxetine []. While some studies suggest a potential correlation between P300 changes and clinical improvement, further research is needed to validate its reliability as a biomarker for treatment response.
Q18: What analytical techniques have been employed to measure Fluoxetine and norfluoxetine concentrations in biological samples?
A26: Various analytical methods have been used to quantify Fluoxetine and norfluoxetine in biological matrices like plasma or serum. Gas chromatography-mass spectrometry (GC/MS) and liquid chromatography-tandem mass spectrometry (LC/MS/MS) are highly sensitive and specific techniques frequently employed for this purpose []. These methods allow for accurate quantification of drug and metabolite concentrations, aiding in pharmacokinetic studies and therapeutic drug monitoring.
Q19: What other analytical techniques have been employed in Fluoxetine research?
A27: Apart from measuring drug concentrations, other analytical techniques have been crucial in Fluoxetine research. For instance, Western blotting has been used to quantify protein expression levels, such as phosphorylated microtubule-associated protein-2 (pMAP-2) in the amygdala of rats, providing insights into the molecular mechanisms underlying Fluoxetine's effects []. Similarly, reverse transcription-polymerase chain reaction (RT-PCR) has been utilized to quantify mRNA levels of specific genes, like GDNF and those involved in serotonergic signaling, furthering our understanding of Fluoxetine's impact on gene expression [, ].
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
![[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl [hydroxy(phosphonooxy)phosphinothioyl] hydrogen phosphate](/img/structure/B1211803.png)