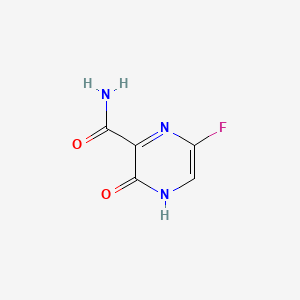
Favipiravir
概要
説明
ファビピラビルは、6-フルオロ-3-ヒドロキシピラジン-2-カルボキサミドとしても知られており、主に日本のインフルエンザ治療に使用される抗ウイルス薬です。富山化学工業株式会社によって開発されました。 そして、エボラ、ラッサ、そして最近ではCOVID-19など、さまざまなRNAウイルス感染症の治療の可能性を示しています 。 ファビピラビルはピラジンカルボキサミド誘導体であり、ウイルスRNA依存性RNAポリメラーゼを阻害することにより、ウイルスの複製を防ぎます .
作用機序
ファビピラビルは、活性型であるファビピラビル-リボフラノシル-5'-トリリン酸(ファビピラビル-RTP)に代謝されるプロドラッグです。 この活性型は、ウイルスの複製に不可欠な酵素であるウイルスRNA依存性RNAポリメラーゼ(RdRp)を阻害します 。 ファビピラビル-RTPは、グアノシンとアデノシンを模倣することで、ウイルスRNAに組み込まれ、RNA合成の早期終結を引き起こします .
6. 類似の化合物との比較
ファビピラビルは、ウイルスRNAポリメラーゼを標的とするレムデシビルやリバビリンなどの他の抗ウイルス化合物に似ています。 ファビピラビルの独自のピラジンカルボキサミド構造と、幅広いRNAウイルスを阻害する能力は、それを際立たせています 。 その他の類似の化合物には、構造的に類似しているが、抗ウイルス活性スペクトルが異なるT-1105とT-1106があります .
ファビピラビルの広域スペクトルの抗ウイルス活性、独自の作用機序、およびさまざまなウイルス感染症の治療の可能性は、抗ウイルス研究と治療における重要な化合物として位置づけています。
生化学分析
Biochemical Properties
Favipiravir functions as a prodrug and undergoes ribosylation and phosphorylation intracellularly to become the active form, this compound-ribofuranosyl-5’-triphosphate (this compound-RTP) . This compound-RTP binds to and inhibits RNA dependent RNA polymerase (RdRp), which ultimately prevents viral transcription and replication . It mimics both guanosine and adenosine for the viral RdRP .
Cellular Effects
This compound has shown to have significant effects on viral clearance and clinical improvement in patients with COVID-19 . It induces viral clearance by 7 days and contributes to clinical improvement within 14 days . This compound significantly improved the clinical symptoms of infected patients, reduced viral titer and RNA copies in serum, and extended overall survival .
Molecular Mechanism
This compound functions as a prodrug and undergoes ribosylation and phosphorylation intracellularly to become the active this compound-RTP . This compound-RTP binds to and inhibits RNA dependent RNA polymerase (RdRp), which ultimately prevents viral transcription and replication . This compound-RTP is a nucleoside analogue. It mimics both guanosine and adenosine for the viral RdRP .
Temporal Effects in Laboratory Settings
In early symptomatic COVID-19 treatment, high dose oral this compound did not accelerate viral clearance . This compound induces viral clearance by 7 days and contributes to clinical improvement within 14 days . The results indicated that this compound has strong possibility for treating COVID-19, especially in patients with mild-to-moderate illness .
Dosage Effects in Animal Models
In animal models, this compound significantly reduced virus titers 10- to 100-fold in sera at all three time points compared to vehicle-treated mice . And this compound treatment effectively reduced the virus copies by approximately 10-fold across the three time points, relative to vehicle-treated mice .
Metabolic Pathways
This compound is extensively metabolized with metabolites excreted mainly in the urine . The antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to the inactive metabolite, T705M1 .
Transport and Distribution
The apparent volume of distribution of this compound is 15 - 20 L . This compound is 54% plasma protein-bound . Of this fraction, 65% is bound to serum albumin and 6.5% is bound to ɑ1-acid glycoprotein .
Subcellular Localization
Given its mechanism of action, it can be inferred that this compound, after being converted to its active form this compound-RTP in the cytoplasm, likely localizes to the site of viral replication within the host cell, where it inhibits the viral RNA-dependent RNA polymerase .
準備方法
ファビピラビルは、いくつかの経路を通じて合成することができます。一般的な方法の1つは、2-アミノピラジンから始まり、位置選択的な塩素化、臭素化、およびパラジウム触媒によるシアノ化を受けて、3,6-ジクロロピラジン-2-カルボニトリルを形成します。 この中間体を次に、求核的フッ素化、ニトリル水和、およびヒドロキシル置換にかけると、ファビピラビルが得られます 。 別のスケーラブルな合成では、マロン酸ジエチルを出発物質として使用し、連続フローリアクターで水素化と臭素化を行い、16%の収率で得られます .
化学反応の分析
ファビピラビルは、以下を含むさまざまな化学反応を起こします。
フッ素化: ピラジン環へのフッ素原子の導入。
水酸化: ヒドロキシル基の付加。
ニトリル加水分解: ニトリルからカルボキサミドへの変換。これらの反応で一般的に使用される試薬には、フッ素化用のフッ化水素と、ニトリル加水分解用の過酸化水素などがあります。
科学的研究の応用
ファビピラビルは、その抗ウイルス特性について広く研究されています。 インフルエンザ、エボラ、SARS-CoV-2など、幅広いRNAウイルスに対して有効であることが示されています 。 ウイルス感染症の治療に加えて、ファビピラビルはラッサ熱やジカウイルスなど、他の疾患の治療における可能性について調査されています 。その広域スペクトルの抗ウイルス活性は、臨床および研究設定の両方において貴重なツールとなっています。
類似化合物との比較
Favipiravir is similar to other antiviral compounds like remdesivir and ribavirin, which also target viral RNA polymerase. this compound’s unique pyrazinecarboxamide structure and its ability to inhibit a broad spectrum of RNA viruses set it apart . Other similar compounds include T-1105 and T-1106, which share structural similarities but differ in their antiviral activity spectrum .
This compound’s broad-spectrum antiviral activity, unique mechanism of action, and potential for treating various viral infections make it a significant compound in antiviral research and therapy.
特性
IUPAC Name |
5-fluoro-2-oxo-1H-pyrazine-3-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C5H4FN3O2/c6-2-1-8-5(11)3(9-2)4(7)10/h1H,(H2,7,10)(H,8,11) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZCGNOVWYSGBHAU-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=C(N=C(C(=O)N1)C(=O)N)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C5H4FN3O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60948878 | |
| Record name | 6-Fluoro-3-hydroxypyrazine-2-carboxamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60948878 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
157.10 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
slightly soluble in water | |
| Record name | Favipiravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12466 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
The mechanism of action of favipiravir is novel compared to existing influenza antivirals that primarily prevent entry and exit of the virus from cells. The active favipiravir-RTP selectively inhibits RNA polymerase and prevents replication of the viral genome. There are several hypotheses as to how favipiravir-RTP interacts with RNA dependent RNA polymerase (RdRp). Some studies have shown that when favipiravir-RTP is incorporated into a nascent RNA strand, it prevents RNA strand elongation and viral proliferation. Studies have also found that the presence of purine analogs can reduce favipiravir’s antiviral activity, suggesting competition between favipiravir-RTP and purine nucleosides for RdRp binding. Although favipiravir was originally developed to treat influenza, the RdRp catalytic domain (favipiravir's primary target), is expected to be similar for other RNA viruses. This conserved RdRp catalytic domain contributes to favipiravir's broad-spectrum coverage. | |
| Record name | Favipiravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12466 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
259793-96-9 | |
| Record name | Favipiravir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=259793-96-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Favipiravir [USAN:INN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0259793969 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Favipiravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12466 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | 6-Fluoro-3-hydroxypyrazine-2-carboxamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60948878 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | FAVIPIRAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/EW5GL2X7E0 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
187℃ to 193℃ | |
| Record name | Favipiravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12466 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Favipiravir?
A1: this compound is a broad-spectrum antiviral drug that acts as a selective inhibitor of RNA-dependent RNA polymerase (RdRp), an enzyme crucial for viral RNA replication and transcription. []
Q2: How does this compound interact with RdRp?
A2: this compound is a prodrug that is metabolized intracellularly into its active form, this compound ribofuranosyl-5’-triphosphate (FAVI-RTP). FAVI-RTP acts as a purine analogue and competes with both guanine and adenine for binding to RdRp. This competition leads to the inhibition of viral RNA synthesis. [, , , ]
Q3: Does this compound induce lethal mutagenesis in viruses?
A3: While initially thought to be a chain terminator, recent studies suggest this compound can induce lethal mutagenesis in some viruses, like influenza A H1N1, by increasing the mutation rate in viral RNA beyond a sustainable threshold. []
Q4: What are the downstream effects of this compound inhibiting RdRp?
A4: Inhibition of RdRp by this compound leads to a decrease in viral RNA synthesis, ultimately hindering the replication and transcription processes essential for viral propagation. This results in a reduction of viral load and potentially limits the severity of viral infection. [, , ]
Q5: What is the molecular formula and weight of this compound?
A6: The molecular formula of this compound is C5H4FN3O2, and its molecular weight is 157.1 g/mol. []
Q6: What are the pharmacokinetic properties of this compound?
A8: this compound is a prodrug that is rapidly absorbed after oral administration and is metabolized primarily by aldehyde oxidase (AO) to its active form, FAVI-RTP. [] It exhibits a relatively short half-life in plasma, but its active metabolite, FAVI-RTP, has a longer intracellular half-life. [] This discrepancy suggests that while this compound is cleared rapidly from the plasma, it might maintain sufficient intracellular concentrations of FAVI-RTP for a longer duration. []
Q7: Does the presence of other drugs influence the pharmacokinetics of this compound?
A9: Yes, co-administration of this compound with drugs metabolized by or affecting AO or xanthine oxidase (XO) can alter its pharmacokinetic profile. For instance, co-administration with pioglitazone, an AO substrate, altered this compound's pharmacokinetics. Similarly, interactions were observed with citalopram (an AO substrate and modulator) and allopurinol (an AO substrate and XO inhibitor). [, ]
Q8: What is the relationship between this compound plasma concentrations and its antiviral effect?
A10: In a study on uncomplicated influenza, achieving an average minimum plasma concentration (Cmin) of this compound greater than 20 μg/mL was associated with enhanced antiviral activity, faster viral clearance, and quicker alleviation of symptoms compared to lower concentrations or placebo. []
Q9: Has resistance to this compound been observed in viruses?
A13: Yes, although this compound was initially thought to have a high barrier to resistance, studies have shown that resistance can develop, albeit at a lower rate compared to other antiviral drugs. [, ]
Q10: Which mutations are associated with this compound resistance?
A14: In influenza A virus, a key mutation associated with this compound resistance is K229R in the PB1 subunit of RdRp. [] This mutation confers resistance in vitro and in cell culture but comes at a cost to viral fitness. A compensatory mutation, P653L in the PA subunit, can restore viral fitness. [] Interestingly, the location of the K229R mutation within a conserved motif of RdRp raises the possibility that other RNA viruses might develop resistance through similar mutations. []
Q11: What are the known adverse effects associated with this compound?
A16: this compound has been associated with various adverse effects, including gastrointestinal disturbances, elevated liver enzymes, hyperuricemia, and potential teratogenicity. [, , , , , ] The incidence and severity of these effects can vary based on factors such as dosage, duration of treatment, and individual patient characteristics.
Q12: What toxicological effects has this compound demonstrated in preclinical studies?
A17: Preclinical studies in rats have shown that this compound administration, particularly at high doses, can induce oxidative stress, inflammation, and histopathological changes in the liver, kidneys, and lungs. [, , ] These findings underscore the importance of careful monitoring for potential toxicity in clinical settings.
Q13: What alternative routes of administration are being explored for this compound?
A18: To potentially improve drug delivery directly to the lungs, researchers are developing an inhalation solution of this compound. [] This approach aims to enhance drug concentration at the site of infection in respiratory illnesses like COVID-19.
Q14: What analytical methods are commonly used to quantify this compound?
A19: High-performance liquid chromatography (HPLC) coupled with UV or tandem mass spectrometry (HPLC-MS/MS) are frequently used for the accurate and sensitive quantification of this compound in biological matrices. [, , ]
Q15: Have any analytical methods been developed for monitoring this compound treatment?
A20: While specific biomarkers for monitoring this compound treatment are still under investigation, next-generation sequencing (NGS) has been proposed as a tool to track the drug's mutagenic effect on viruses. [] By analyzing the pattern of mutations induced by this compound, researchers can potentially assess its antiviral activity and monitor for the emergence of resistance. []
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![2-Phenyl-3,10-diazatricyclo[6.4.1.04,13]trideca-1,4,6,8(13)-tetraen-9-one](/img/structure/B1662715.png)