モキシフロキサシン
概要
説明
Moxifloxacin is a synthetic fluoroquinolone antibiotic used to treat a variety of bacterial infections. It is effective against both Gram-positive and Gram-negative bacteria. Moxifloxacin is commonly used to treat respiratory tract infections, skin infections, and intra-abdominal infections. It is available in oral, intravenous, and ophthalmic formulations .
作用機序
モキシフロキサシンは、トポイソメラーゼII(DNAジャイレース)とトポイソメラーゼIVの酵素を阻害することにより、抗菌作用を発揮します。これらの酵素は、細菌のDNA複製、転写、修復に不可欠です。 これらの酵素を阻害することにより、モキシフロキサシンは細菌がDNAを複製および修復するのを阻止し、細菌細胞の死滅につながります .
科学的研究の応用
Moxifloxacin has a wide range of scientific research applications:
Chemistry: Used as a model compound in studies of quinolone synthesis and reactivity.
Biology: Studied for its effects on bacterial DNA replication and repair mechanisms.
Medicine: Extensively researched for its efficacy in treating various bacterial infections, including respiratory tract infections, skin infections, and intra-abdominal infections.
Industry: Used in the development of new antibiotic formulations and drug delivery systems .
生化学分析
Biochemical Properties
Moxifloxacin’s role in biochemical reactions primarily involves the inhibition of bacterial enzymes. It inhibits the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV, which are essential for bacterial DNA replication and transcription . By inhibiting these enzymes, moxifloxacin prevents bacteria from duplicating their DNA, thereby stopping their growth and proliferation .
Cellular Effects
Moxifloxacin has been shown to have significant effects on various types of cells and cellular processes. For instance, it has been found to inhibit the yeast to Hyphal morphogenesis by affecting signaling pathways . It also arrested the cell cycle of Candida albicans at the S phase . Moreover, it has been observed to have photostability and reduced phototoxicity on melanocytes .
Molecular Mechanism
The molecular mechanism of action of moxifloxacin involves its interaction with bacterial enzymes. It binds to and inhibits the activity of DNA gyrase and topoisomerase IV . These enzymes are responsible for maintaining the superhelical structure of DNA and are required for DNA replication and transcription, DNA repair, recombination, and transposition . By inhibiting these enzymes, moxifloxacin prevents the bacteria from duplicating their DNA, leading to their death .
Temporal Effects in Laboratory Settings
In laboratory settings, moxifloxacin has shown a dose-dependent reduction in bacterial density over time . It has been found to be the most effective among the four fluoroquinolones tested against penicillin-resistant streptococcal pneumonia in a mouse model . Moreover, it has been observed that moxifloxacin has a good stability profile, with no significant degradation over time .
Dosage Effects in Animal Models
In animal models, the effects of moxifloxacin have been observed to vary with different dosages. In rabbit models of meningitis, treatment with moxifloxacin resulted in a dose-dependent reduction in bacterial density . In a murine model of disseminated Mycobacterium tuberculosis infection, moxifloxacin was equivalent in its ability to prevent mortality and reduce the concentration of bacteria in spleens to other antibiotics tested .
Metabolic Pathways
Moxifloxacin undergoes Phase II metabolism in humans, resulting in the formation of two main metabolites: N-sulphate (M1) and acylglucuronide (M2) . These metabolites are pharmacologically inactive . Moxifloxacin is eliminated via metabolic, renal, and biliary/faecal routes .
Transport and Distribution
Moxifloxacin is rapidly absorbed in humans, with a high bioavailability of approximately 90% . It has a good distribution to saliva, interstitial fluids, and lung tissues . About 22% of a moxifloxacin dose is excreted via the kidneys as unmetabolised moxifloxacin .
準備方法
Synthetic Routes and Reaction Conditions: Moxifloxacin is synthesized through a multi-step process. The key steps involve the formation of the quinolone core, followed by the introduction of the cyclopropyl group, the methoxy group, and the diazabicyclononane moiety. The synthesis typically involves the following steps:
- Formation of the quinolone core through a condensation reaction between an aniline derivative and a β-ketoester.
- Introduction of the cyclopropyl group via a cyclopropanation reaction.
- Addition of the methoxy group through a nucleophilic substitution reaction.
- Formation of the diazabicyclononane moiety through a cyclization reaction .
Industrial Production Methods: Industrial production of moxifloxacin involves large-scale synthesis using optimized reaction conditions to ensure high yield and purity. The process includes:
- Use of high-purity starting materials and reagents.
- Optimization of reaction conditions such as temperature, pressure, and pH.
- Implementation of purification techniques such as crystallization and chromatography to obtain the final product .
化学反応の分析
反応の種類: モキシフロキサシンは、以下を含む様々な化学反応を起こします。
酸化: モキシフロキサシンは、酸化されてキノロンN-オキシドを生成する可能性があります。
還元: モキシフロキサシンの還元は、ジヒドロキノロン誘導体の生成につながる可能性があります。
一般的な試薬と条件:
酸化: 一般的な酸化剤には、過酸化水素と過マンガン酸カリウムがあります。
還元: 水素化ホウ素ナトリウムや水素化リチウムアルミニウムなどの還元剤が使用されます。
主な生成物:
酸化: キノロンN-オキシド。
還元: ジヒドロキノロン誘導体。
4. 科学研究への応用
モキシフロキサシンは、幅広い科学研究への応用があります。
化学: キノロン合成と反応性の研究におけるモデル化合物として使用されます。
生物学: 細菌のDNA複製と修復メカニズムへの影響が研究されています。
医学: 呼吸器感染症、皮膚感染症、腹腔内感染症など、様々な細菌感染症の治療における有効性について、広く研究されています。
類似化合物との比較
モキシフロキサシンは、シプロフロキサシンやレボフロキサシンなどの他のフルオロキノロン系抗生物質と比較されます。
類似化合物:
- シプロフロキサシン
- レボフロキサシン
- オフロキサシン
- ノルフロキサシン
モキシフロキサシンは、幅広い活性スペクトルと優れた組織浸透性など、独自の特性を備えているため、様々な細菌感染症の治療に有効な抗生物質です。
特性
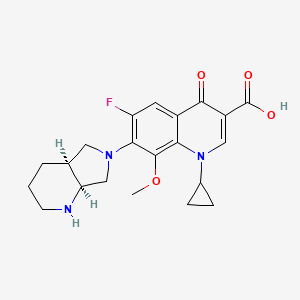
IUPAC Name |
7-[(4aS,7aS)-1,2,3,4,4a,5,7,7a-octahydropyrrolo[3,4-b]pyridin-6-yl]-1-cyclopropyl-6-fluoro-8-methoxy-4-oxoquinoline-3-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H24FN3O4/c1-29-20-17-13(19(26)14(21(27)28)9-25(17)12-4-5-12)7-15(22)18(20)24-8-11-3-2-6-23-16(11)10-24/h7,9,11-12,16,23H,2-6,8,10H2,1H3,(H,27,28)/t11-,16+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FABPRXSRWADJSP-MEDUHNTESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C2C(=CC(=C1N3CC4CCCNC4C3)F)C(=O)C(=CN2C5CC5)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COC1=C2C(=CC(=C1N3C[C@@H]4CCCN[C@@H]4C3)F)C(=O)C(=CN2C5CC5)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H24FN3O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3048491 | |
| Record name | Moxifloxacin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3048491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
401.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Moxifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
1.68e-01 g/L | |
| Record name | Moxifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The bactericidal action of moxifloxacin results from inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division., The fluoroquinolone antibiotic moxifloxacin has been associated with the acquired long QT syndrome and is used as a positive control in the evaluation of the QT-interval prolonging potential of new drugs. In common with other QT-prolonging agents, moxifloxacin is known to inhibit the hERG potassium K+ channel, but at present there is little mechanistic information available on this action. This study was conducted in order to characterise the inhibition of hERG current (I(hERG)) by moxifloxacin, and to determine the role in drug binding of the S6 aromatic amino-acid residues Tyr652 and Phe656. hERG currents were studied using whole-cell patch clamp (at room temperature and at 35-37 degrees C) in an HEK293 cell line stably expressing hERG channels. Moxifloxacin reversibly inhibited currents in a dose-dependent manner. We investigated the effects of different voltage commands to elicit hERG currents on moxifloxacin potency. Using a 'step-ramp' protocol, the IC50 was 65 uM at room temperature and 29 microM at 35 degrees C. When a ventricular action potential waveform was used to elicit currents, the IC50 was 114 microM. Block of hERG by moxifloxacin was found to be voltage-dependent, occurred rapidly and was independent of stimulation frequency. Mutagenesis of the S6 helix residue Phe656 to Ala failed to eliminate or reduce the moxifloxacin-mediated block whereas mutation of Tyr652 to Ala reduced moxifloxacin block by approximately 66%. Our data demonstrate that moxifloxacin blocks the hERG channel with a preference for the activated channel state. The Tyr652 but not Phe656 S6 residue is involved in moxifloxacin block of hERG, concordant with an interaction in the channel inner cavity., The bactericidal action of moxifloxacin results from inhibition of the topoisomerase II (DNA gyrase) and topoisomerase IV required for bacterial DNA replication, transcription, repair, and recombination. It appears that the C8-methoxy moiety contributes to enhanced activity and lower selection of resistant mutants of Gram-positive bacteria compared to the C8-H moiety. The presence of the bulky bicycloamine substituent at the C-7 position prevents active efflux, associated with the NorA or pmrA genes seen in certain Gram-positive bacteria., Torsade de pointes (TdP) is increasingly recognized as a complication of drug therapy. The most common cause of drug-induced QT prolongation is inhibition of the rapidly activating component of the delayed potassium current (I(Kr)). Moxifloxacin, a widely used fluoroquinolone, is a weak I(Kr) inhibitor and has been associated with QT prolongation., Fluoroquinolones prolong the QT interval by blocking voltage-gated potassium channels, especially the rapid component of the delayed rectifier potassium current I(Kr), expressed by HERG (the human ether-a-go-go-related gene). According to the available case reports and clinical studies, moxifloxacin carries the greatest risk of QT prolongation from all available quinolones in clinical practice and it should be used with caution in patients with predisposing factors for Torsades de pointes (TdP). | |
| Record name | Moxifloxacin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00218 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Moxifloxacin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
151096-09-2, 354812-41-2 | |
| Record name | Moxifloxacin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=151096-09-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Moxifloxacin [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0151096092 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Moxifloxacin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00218 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Moxifloxacin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3048491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.129.459 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MOXIFLOXACIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/U188XYD42P | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Moxifloxacin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Moxifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
238-242 °C, 208-209 °C (decomposes), 238 - 242 °C | |
| Record name | Moxifloxacin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00218 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Moxifloxacin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Moxifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![[4-chloro-3-[5-methyl-3-[4-(2-pyrrolidin-1-ylethoxy)anilino]-1,2,4-benzotriazin-7-yl]phenyl] benzoate;hydrochloride](/img/structure/B1663545.png)