アミロライド
概要
説明
Amiloride is a pyrazine derivative that is widely used as a potassium-sparing diuretic. It is primarily employed in the treatment of hypertension and congestive heart failure. Amiloride works by inhibiting sodium reabsorption in the kidneys, which helps to reduce fluid retention and lower blood pressure .
科学的研究の応用
Amiloride has a wide range of scientific research applications, including:
Chemistry: Used as a reagent in various chemical reactions and studies involving ion channels.
Biology: Employed in studies of cellular ion transport and signaling pathways.
Medicine: Investigated for its potential anti-cancer properties and its role in treating conditions like cystic fibrosis and chronic obstructive pulmonary disease.
Industry: Used in the development of new pharmaceuticals and as a tool in drug discovery
作用機序
Target of Action
Amiloride primarily targets the amiloride-sensitive sodium channels located in the distal convoluted tubules and collecting ducts in the kidneys . These channels play a crucial role in sodium reabsorption, which is essential for maintaining electrolyte balance and blood pressure .
Mode of Action
Amiloride interacts with its targets by binding to the amiloride-sensitive sodium channels, thereby inhibiting sodium reabsorption . This inhibition creates a negative potential in the luminal membranes of principal cells, reducing the secretion of potassium and hydrogen ions . The overall effect is a promotion of the loss of sodium and water from the body, without depleting potassium .
Biochemical Pathways
The inhibition of sodium reabsorption by amiloride affects the sodium-potassium balance in the body. It disrupts the normal function of the sodium/potassium ATPase pump, which maintains the necessary electrochemical gradients . This disruption leads to an increase in sodium and water excretion while reducing potassium excretion .
Pharmacokinetics
Amiloride is readily absorbed and begins to act within 2 hours after an oral dose . Its effect on electrolyte excretion reaches a peak between 6 and 10 hours and lasts about 24 hours . Peak plasma levels are obtained in 3 to 4 hours, and the plasma half-life varies from 6 to 9 hours .
Result of Action
The molecular and cellular effects of amiloride’s action include a decrease in sodium reabsorption and an increase in sodium and water excretion . This results in a reduction in potassium and hydrogen ion secretion . At the cellular level, amiloride can cause changes in cell volume and electrolyte concentrations .
Action Environment
Environmental factors can influence the action, efficacy, and stability of amiloride. For instance, the presence of other medications, the patient’s diet, and the patient’s health status (such as kidney function) can all impact how amiloride works in the body . .
生化学分析
Biochemical Properties
Amiloride works by inhibiting sodium reabsorption in the distal convoluted tubules and collecting ducts in the kidneys by binding to the amiloride-sensitive sodium channels . This promotes the loss of sodium and water from the body, but without depleting potassium .
Cellular Effects
Amiloride has been shown to have significant effects on various types of cells. It can cause changes to the levels of certain minerals in your body, called electrolytes. For example, it may cause high potassium levels (hyperkalemia), low sodium levels (hyponatremia), or low chloride levels (hypochloremia) .
Molecular Mechanism
Amiloride exerts its effects at the molecular level by binding to the amiloride-sensitive sodium channels in the distal convoluted tubules and collecting ducts of the nephron . This binding inhibits sodium reabsorption, which in turn reduces the secretion of potassium and hydrogen ions .
Temporal Effects in Laboratory Settings
Amiloride is administered orally and reaches its peak diuretic effect at 6–10 hours . It does not undergo metabolism by the liver and is predominantly excreted unchanged in urine and faeces .
Dosage Effects in Animal Models
In animal models, such as dogs and cats, the dosage of Amiloride is typically around 0.1 mg/kg p.o. q12h . The effects of Amiloride can vary with different dosages, and high doses may lead to adverse effects such as hyperkalemia .
Metabolic Pathways
Amiloride does not undergo metabolism by the liver and is predominantly excreted unchanged in urine and faeces . It acts independently of aldosterone, inhibiting sodium reabsorption through epithelial sodium channels (ENaC) and preventing potassium excretion in the distal convoluted tubule and collecting ducts .
Transport and Distribution
Amiloride is transported and distributed within cells and tissues via the bloodstream. It acts at the distal convoluted tubule and collecting duct of the nephron to produce its diuretic effect .
Subcellular Localization
The subcellular localization of Amiloride is primarily at the distal convoluted tubules and collecting ducts of the nephron . It binds to the amiloride-sensitive sodium channels in these areas, inhibiting sodium reabsorption and reducing the secretion of potassium and hydrogen ions .
準備方法
Synthetic Routes and Reaction Conditions: Amiloride can be synthesized through a multi-step process involving the reaction of 3,5-diamino-6-chloropyrazine-2-carboxamide with various reagents. One common method involves the reaction of 3,5-diamino-6-chloropyrazine-2-carboxamide with cyanamide under acidic conditions to form amiloride .
Industrial Production Methods: Industrial production of amiloride typically involves large-scale synthesis using similar reaction conditions as in laboratory synthesis. The process is optimized for yield and purity, often involving purification steps such as recrystallization and chromatography to ensure the final product meets pharmaceutical standards .
化学反応の分析
Types of Reactions: Amiloride undergoes several types of chemical reactions, including:
Oxidation: Amiloride can be oxidized under specific conditions, although this is not a common reaction in its typical use.
Reduction: Reduction reactions are less common for amiloride due to its stable structure.
Substitution: Amiloride can undergo substitution reactions, particularly at the amino groups.
Common Reagents and Conditions:
Oxidation: Strong oxidizing agents can be used, but these reactions are not typically relevant to its pharmacological use.
Substitution: Reagents such as alkyl halides can be used for substitution reactions at the amino groups under basic conditions.
Major Products Formed: The major products formed from these reactions depend on the specific reagents and conditions used. For example, substitution reactions can yield various alkylated derivatives of amiloride .
類似化合物との比較
Triamterene: Another potassium-sparing diuretic with a similar mechanism of action.
Spironolactone: A potassium-sparing diuretic that works by antagonizing aldosterone receptors.
Eplerenone: Similar to spironolactone but with fewer side effects.
Uniqueness of Amiloride: Amiloride is unique in its specific inhibition of the ENaC, which makes it particularly effective in conditions where sodium retention is a problem. Unlike spironolactone and eplerenone, amiloride does not affect aldosterone receptors, which reduces the risk of hormonal side effects .
特性
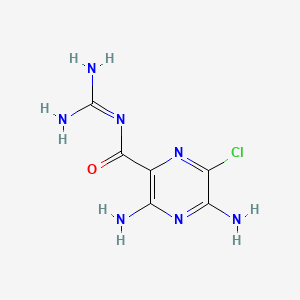
IUPAC Name |
3,5-diamino-6-chloro-N-(diaminomethylidene)pyrazine-2-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XSDQTOBWRPYKKA-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1(=C(N=C(C(=N1)Cl)N)N)C(=O)N=C(N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C6H8ClN7O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
17440-83-4 (hydrochloride), 2016-88-8 (anhydrous hydrochloride) | |
| Record name | Amiloride [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002609463 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID9043853 | |
| Record name | Amiloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9043853 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
229.63 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Amiloride | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014732 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Slightly soluble, 1.22e+00 g/L | |
| Record name | Amiloride | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00594 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Amiloride | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014732 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Amiloride works by inhibiting sodium reabsorption in the distal convoluted tubules and collecting ducts in the kidneys by binding to the amiloride-sensitive sodium channels. This promotes the loss of sodium and water from the body, but without depleting potassium. Amiloride exerts its potassium sparing effect through the inhibition of sodium reabsorption at the distal convoluted tubule, cortical collecting tubule and collecting duct; this decreases the net negative potential of the tubular lumen and reduces both potassium and hydrogen secretion and their subsequent excretion. Amiloride is not an aldosterone antagonist and its effects are seen even in the absence of aldosterone. | |
| Record name | Amiloride | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00594 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
2609-46-3, 2016-88-8 | |
| Record name | Amiloride | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=2609-46-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Amiloride [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002609463 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Amiloride | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00594 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Amiloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9043853 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Amiloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.018.205 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | AMILORIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/7DZO8EB0Z3 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Amiloride | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014732 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
240.5-241.5, 240 °C | |
| Record name | Amiloride | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00594 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Amiloride | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014732 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary molecular target of amiloride?
A1: Amiloride primarily targets the epithelial sodium channel (ENaC) [, , , , , ]. This channel plays a crucial role in sodium reabsorption in various epithelia, including those in the kidneys, lungs, and colon.
Q2: How does amiloride interact with ENaC?
A2: Amiloride binds to the extracellular pore of ENaC, blocking the influx of sodium ions [, , ]. This binding is thought to be voltage-dependent, with a more positive apical membrane potential reducing amiloride's affinity for the channel [].
Q3: Does amiloride affect other ion transport systems?
A3: Yes, amiloride has been shown to interact with other sodium transporters and channels, albeit with lower affinity compared to ENaC. These include Na+/H+ exchangers [, , , ], Na+/Ca2+ exchangers [], and even α-adrenergic receptors [, ].
Q4: What are the downstream effects of amiloride's interaction with ENaC?
A4: By blocking ENaC, amiloride reduces sodium reabsorption in epithelia. This leads to increased sodium excretion in the urine, promoting diuresis [, , ]. The potassium-sparing effect arises from reduced sodium delivery to the collecting duct, where sodium reabsorption is coupled with potassium secretion [, , ].
Q5: Does amiloride have any effects on intracellular pH?
A5: Yes, amiloride can indirectly affect intracellular pH (pHi) by inhibiting Na+/H+ exchangers. These exchangers typically extrude protons from the cell in exchange for sodium. Inhibiting this process can lead to intracellular acidification [, ].
Q6: What is the molecular formula and weight of amiloride?
A6: The molecular formula of amiloride hydrochloride is C6H8ClN7O•HCl, and its molecular weight is 267.69 g/mol.
Q7: Are there any specific structural features of amiloride crucial for its activity?
A7: Yes, the unsubstituted guanidino group is essential for amiloride's inhibitory action on the Na+/H+ exchanger []. Modifications to this group dramatically reduce its potency.
Q8: Do structural changes affect amiloride's selectivity for different targets?
A9: Yes, different amiloride analogs display varying affinities for ENaC and other ion transporters [, , , ]. For instance, benzamil exhibits higher selectivity for ENaC compared to amiloride, while ethylisopropylamiloride shows greater potency for blocking the Na+/H+ exchanger [, ].
Q9: How is amiloride typically administered, and what is its absorption profile?
A10: Amiloride is usually administered orally. It exhibits relatively good absorption from the gastrointestinal tract, but its bioavailability is limited due to significant first-pass metabolism [].
Q10: What is the primary route of amiloride elimination?
A11: Amiloride is primarily excreted unchanged in the urine [].
Q11: Are there any known mechanisms of resistance to amiloride?
A14: While specific resistance mechanisms haven't been extensively studied, alterations in ENaC expression or mutations in its structure could potentially lead to reduced amiloride sensitivity [].
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。