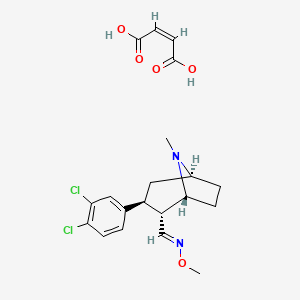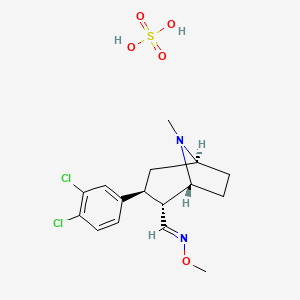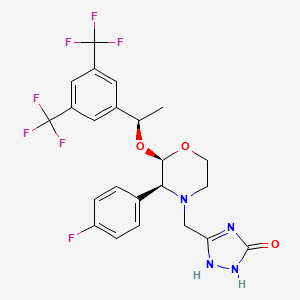
アプレピタント
概要
説明
科学的研究の応用
Aprepitant has a wide range of scientific research applications, particularly in the fields of chemistry, biology, medicine, and industry . In chemistry, it is used as a model compound for studying the synthesis and characterization of neurokinin-1 receptor antagonists . In biology, aprepitant is used to investigate the role of substance P in various physiological and pathological processes . In medicine, it is widely used to prevent nausea and vomiting in patients undergoing chemotherapy and surgery . Additionally, aprepitant is being explored for its potential use in treating other conditions, such as depression and anxiety .
作用機序
生化学分析
Biochemical Properties
Aprepitant interacts with the NK1 receptors, blocking the signals given off by these receptors . It has little or no affinity for serotonin (5-HT3), dopamine, and corticosteroid receptors .
Cellular Effects
Aprepitant has been shown to inhibit the proliferation, migration, and invasion of gallbladder cancer cells . It also significantly boosts the apoptosis, reactive oxygen species (ROS), and inflammation response in gallbladder cancer .
Molecular Mechanism
Aprepitant works by blocking substance P from attaching to the NK1 receptors . This decreases the likelihood of vomiting in patients . It is classified as an NK1 antagonist because it blocks signals given off by NK1 receptors .
Temporal Effects in Laboratory Settings
Aprepitant provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy . It has been shown to enhance control of chemotherapy-induced nausea and vomiting (CINV) over time .
Dosage Effects in Animal Models
In animal models, aprepitant has been shown to have anti-inflammatory and antioxidant properties . The effects of aprepitant on the lung tissues of rats with an experimental polymicrobial sepsis model were examined, and it was found that aprepitant effectively inhibited the proliferation, migration, and invasion of these cells .
Metabolic Pathways
Aprepitant primarily undergoes CYP3A4-mediated metabolism, as well as minor metabolism mediated by CYP1A2 and CYP2C19 . About seven metabolites of aprepitant have been identified in human plasma, which all retain weak pharmacological activity .
Transport and Distribution
Aprepitant is generally safe and well tolerated in healthy subjects. Its pharmacokinetics is comparable between different ethnicities following single-dose administration . The pharmacokinetics following a clinical 3-day regimen on healthy subjects has been characterized .
準備方法
合成ルートと反応条件: アプレピタントは、いくつかの重要な中間体を用いた複数段階のプロセスによって合成されます。 合成は一般的にモルフォリン誘導体の調製から始まり、その後、アルキル化、アシル化、環化など、様々な化学反応が行われます . 最終生成物は、精製および結晶化工程を経て得られます。これにより、高純度と高収率が保証されます .
工業生産方法: 工業的な設定では、アプレピタントの生産は、収率を最大化し、不純物を最小限に抑えるために反応条件を最適化することを含みます。 高速液体クロマトグラフィー(HPLC)やガスクロマトグラフィー(GC)などの技術を使用して、反応の進行を監視し、最終生成物の品質を確保します . さらに、ナノ沈殿や固体分散などの高度な製剤技術が、アプレピタントの溶解性とバイオアベイラビリティを向上させるために使用されます .
化学反応の分析
反応の種類: アプレピタントは、酸化、還元、置換など、様々な化学反応を起こします . これらの反応は、化合物の化学構造を修飾し、薬理学的特性を改善するために不可欠です。
一般的な試薬と条件: アプレピタントの合成に使用される一般的な試薬には、アルキル化剤、アシル化剤、還元剤などがあります . 反応は一般的に、最適な収率と最小限の副反応を確保するために、特定の温度、圧力、pHレベルなどの制御された条件下で行われます .
生成される主な生成物: アプレピタントの化学反応によって生成される主な生成物には、様々な中間体と最終的な有効成分(API)が含まれます . これらの生成物は、核磁気共鳴(NMR)分光法、質量分析法(MS)、赤外線(IR)分光法などの技術を使用して特性評価され、その構造と純度が確認されます .
科学研究への応用
アプレピタントは、特に化学、生物学、医学、産業の分野において、幅広い科学研究への応用があります . 化学では、神経キニン-1受容体拮抗薬の合成と特性評価を研究するためのモデル化合物として使用されます . 生物学では、アプレピタントは、様々な生理学的および病理学的プロセスにおけるサブスタンスPの役割を調査するために使用されます . 医学では、化学療法や手術を受けている患者の悪心・嘔吐の予防に広く使用されています . さらに、アプレピタントは、うつ病や不安などの他の疾患の治療における可能性についても研究されています .
類似化合物との比較
特性
IUPAC Name |
3-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholin-4-yl]methyl]-1,4-dihydro-1,2,4-triazol-5-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ATALOFNDEOCMKK-OITMNORJSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F)OC2C(N(CCO2)CC3=NNC(=O)N3)C4=CC=C(C=C4)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H](C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F)O[C@@H]2[C@@H](N(CCO2)CC3=NNC(=O)N3)C4=CC=C(C=C4)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H21F7N4O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3049047 | |
| Record name | Aprepitant | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3049047 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
534.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Aprepitant | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014811 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Practically insoluble, 1.94e-02 g/L | |
| Record name | Aprepitant | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00673 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Aprepitant | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014811 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Aprepitant has been shown in animal models to inhibit emesis induced by cytotoxic chemotherapeutic agents, such as cisplatin, via central actions. Animal and human Positron Emission Tomography (PET) studies with Aprepitant have shown that it crosses the blood brain barrier and occupies brain NK1 receptors. Animal and human studies show that Aprepitant augments the antiemetic activity of the 5-HT3-receptor antagonist ondansetron and the corticosteroid ethasone and inhibits both the acute and delayed phases of cisplatin induced emesis. | |
| Record name | Aprepitant | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00673 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
170729-80-3 | |
| Record name | Aprepitant | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=170729-80-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Aprepitant [USAN:INN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0170729803 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Aprepitant | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00673 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Aprepitant | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=748825 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Aprepitant | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3049047 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Aprepitant | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | APREPITANT | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1NF15YR6UY | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Aprepitant | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014811 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of aprepitant?
A1: Aprepitant exerts its antiemetic effect by acting as a selective antagonist of the neurokinin-1 (NK-1) receptor. [, , , , ] This receptor, primarily located in the central nervous system (CNS), is the primary binding site for substance P, a neuropeptide involved in the transmission of nausea and vomiting signals. [, , ] By blocking substance P from binding to NK-1 receptors, aprepitant effectively disrupts the emetic pathway. []
Q2: What makes aprepitant particularly effective against delayed CINV?
A2: Aprepitant demonstrates prolonged NK-1 receptor occupancy in the CNS, lasting at least 48 hours after a single dose of its prodrug fosaprepitant. [, ] This sustained receptor blockade effectively controls delayed CINV, which typically manifests 2 to 5 days post-chemotherapy. []
Q3: Does aprepitant affect gastric emptying?
A3: While aprepitant effectively controls nausea, there is evidence suggesting that it does not significantly accelerate gastric emptying. [] This suggests its antiemetic action is primarily mediated through central NK-1 receptor antagonism rather than by directly influencing gastric motility.
Q4: What is the molecular formula and weight of aprepitant?
A4: Unfortunately, the provided research papers do not explicitly state the molecular formula and weight of aprepitant. For detailed structural information, it is recommended to consult additional resources like PubChem or DrugBank databases.
Q5: What is fosaprepitant, and how does it relate to aprepitant?
A5: Fosaprepitant is a water-soluble prodrug of aprepitant, meaning it is converted into active aprepitant in the body. [, , , , , ] It is administered intravenously and rapidly metabolized into aprepitant via ubiquitous phosphatases within 30 minutes of administration. [, ] This makes it a suitable alternative for patients who have difficulty tolerating oral medications. [, , ]
Q6: How is aprepitant metabolized?
A6: Aprepitant undergoes extensive metabolism, primarily by cytochrome P450 (CYP) 3A4 enzymes. [, , , , ] It is first metabolized into the active N-dealkylated metabolite (ND-AP), which is further converted into its carbonyl form (ND-CAP). []
Q7: Does food intake influence the bioavailability of aprepitant?
A7: Studies indicate that food does not significantly impact the bioavailability of a single oral dose of aprepitant, suggesting dose adjustments based on food intake are unnecessary. []
Q8: What is the relationship between aprepitant exposure and total bilirubin levels?
A8: A study investigating aprepitant pharmacokinetics in cancer patients revealed a significant correlation between the 120-hour area under the curve (AUC) of aprepitant and total bilirubin levels. [] This suggests that liver function may influence aprepitant exposure, but further investigation is needed to fully elucidate this relationship.
Q9: What are the approved clinical applications of aprepitant and fosaprepitant?
A9: Both aprepitant and fosaprepitant are FDA-approved for preventing CINV in patients undergoing highly emetogenic chemotherapy, typically in combination with a corticosteroid (like dexamethasone) and a 5-HT3 receptor antagonist (like ondansetron). [, , , , , , , ]
Q10: Can a single dose of fosaprepitant effectively prevent CINV?
A10: Yes, clinical trials have demonstrated that a single intravenous dose of fosaprepitant (150 mg) is noninferior to the standard 3-day aprepitant regimen for preventing both acute and delayed CINV in patients receiving high-dose cisplatin. [, , ]
Q11: Is aprepitant effective in preventing CINV in pediatric patients?
A11: While further research is necessary, studies suggest that aprepitant, in combination with standard antiemetic therapy, can be effective in reducing CINV in children and adolescents. [, , , ] One study even reported the safe use of aprepitant in children as young as 11 months old. []
Q12: Are there alternative antiemetic regimens to aprepitant for CINV?
A12: A fixed-dose combination of netupitant (another NK-1 receptor antagonist) and palonosetron (a 5-HT3 receptor antagonist), known as NEPA, has shown comparable efficacy to a 3-day aprepitant regimen in preventing CINV associated with moderately emetogenic chemotherapy. []
Q13: Does aprepitant interact with other medications?
A13: Yes, aprepitant is metabolized by CYP3A4 and can both inhibit and induce this enzyme, potentially leading to drug interactions. [, , , , ] For instance, aprepitant can increase the plasma concentrations of dexamethasone and methylprednisolone. [, , ] It can also decrease the plasma concentrations of drugs metabolized by CYP3A4, such as warfarin and hormonal contraceptives. [, , ]
Q14: Does aprepitant interact with vincristine?
A14: While aprepitant does not appear to cause a clinically significant drug interaction with vincristine that leads to early-onset peripheral neuropathy, there seems to be an increased risk of overall chemotherapy-induced peripheral neuropathy (CIPN) with concomitant use. [] Further research is needed to fully understand this potential interaction.
Q15: Does aprepitant affect prednisolone pharmacokinetics?
A15: A study investigating the co-administration of aprepitant with the R-CHOP chemotherapy regimen, which includes prednisolone, found no significant impact of aprepitant on prednisolone pharmacokinetics. [] This is consistent with previous findings that CYP3A4 inhibitors, like ketoconazole and itraconazole, do not affect prednisolone metabolism, unlike their effect on dexamethasone and methylprednisolone. []
Q16: How does aprepitant interact with voriconazole?
A16: Aprepitant initially inhibits voriconazole metabolism due to its triazole ring, but subsequently induces CYP3A4, leading to increased voriconazole metabolism and potentially subtherapeutic drug levels. [] This interaction highlights the importance of monitoring voriconazole levels when co-administered with aprepitant or fosaprepitant.
Q17: Are there any potential antitumor effects of aprepitant?
A17: While currently used as an antiemetic, preclinical studies suggest that aprepitant may possess antitumor properties through various mechanisms, including antiproliferative, antimetastatic, and pro-apoptotic effects. [, ] Further research is warranted to explore these findings and assess the potential of repurposing aprepitant as an anticancer agent.
Q18: Does aprepitant have any immunomodulatory effects?
A18: Research suggests that aprepitant may possess immunomodulatory properties by decreasing the expression of programmed death 1 (PD-1) on CD4+ T cells and reducing plasma levels of substance P and soluble CD163. [] This indicates a potential role of NK-1 receptor antagonism in modulating monocyte activation during HIV infection, warranting further investigation.
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


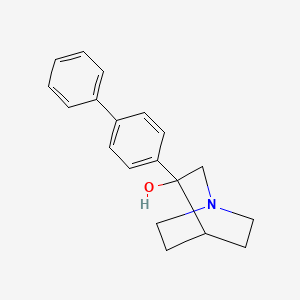
![(E)-1-[(2R,3S)-3-(3,4-Dichlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octan-2-yl]-N-methoxymethanimine](/img/structure/B1667503.png)