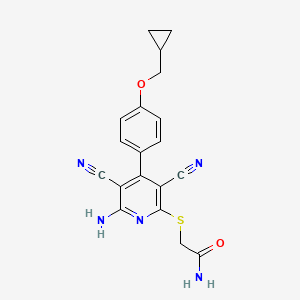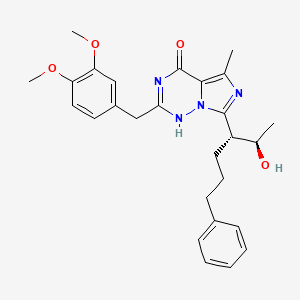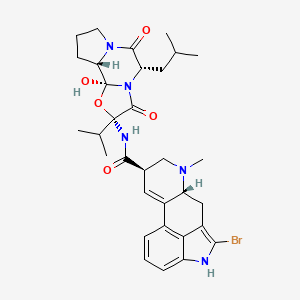
ブロモクリプチン
概要
説明
ブロモクリプチンは、エルゴライン誘導体であり、ドーパミン作動薬です。当初はパーロデルというブランド名で販売されていましたが、その後、さまざまな名称で販売されるようになりました。 ブロモクリプチンは、主に下垂体腫瘍、パーキンソン病、高プロラクチン血症、悪性神経障害症候群、および2型糖尿病の治療に使用されます 。 1968年に特許を取得し、1975年に医療用として承認されました .
科学的研究の応用
Bromocriptine has a wide range of scientific research applications:
Chemistry: It is used as a reference compound in analytical chemistry for the development of new analytical methods.
Biology: Bromocriptine is used to study dopamine receptor functions and signaling pathways.
Medicine: It is extensively used in the treatment of conditions like hyperprolactinemia, Parkinson’s disease, and type 2 diabetes
作用機序
ブロモクリプチンは、ドーパミンD2受容体作動薬として作用することで効果を発揮します。脳内のドーパミン受容体に結合し、下垂体からのプロラクチンの放出を抑制します。この機序は、高プロラクチン血症やパーキンソン病などの病状の治療に役立ちます。 2型糖尿病の場合、ブロモクリプチンは、視床下部のドーパミンレベルを上昇させることで血糖コントロールを改善します。ドーパミンレベルの上昇は、インスリン抵抗性と肝臓のグルコース産生を抑制します .
類似化合物:
カベルゴリン: 高プロラクチン血症とパーキンソン病の治療に使用される別のドーパミン作動薬です。
ペルゴリド: パーキンソン病の治療に使用されます。
比較: ブロモクリプチンは、急速放出製剤という点で独特で、作用開始が速いことが特徴です。また、他のドーパミン作動薬では一般的ではない、2型糖尿病の補助療法としても使用されます。 一方、カベルゴリンは半減期が長く、投与頻度が低いことが好まれる場合が多いです .
生化学分析
Biochemical Properties
Bromocriptine is a partial agonist of the dopamine D2 receptor . It also interacts with other dopamine receptors and with various serotonin and adrenergic receptors . Bromocriptine has additionally been found to inhibit the release of glutamate by reversing the GLT1 glutamate transporter .
Cellular Effects
Bromocriptine blocks the release of a hormone called prolactin from the pituitary gland . Prolactin affects the menstrual cycle and milk production . Bromocriptine is used to treat certain menstrual problems in women and stops milk production in some men and women who have abnormal milk leakage .
Molecular Mechanism
Bromocriptine stimulates centrally-located dopaminergic receptors resulting in a number of pharmacologic effects . Five dopamine receptor types from two dopaminergic subfamilies have been identified . The dopaminergic D1 receptor subfamily consists of D1 and D5 subreceptors, which are associated with dyskinesias .
Temporal Effects in Laboratory Settings
In laboratory settings, bromocriptine use was associated with a modest but statistically significant decline in temperature, with nadir at 72 h post initiation . This suggests that the effects of bromocriptine can change over time in a laboratory setting.
Dosage Effects in Animal Models
In animal models, studies conducted on bromocriptine have shown that stimulating D2 receptors may enhance working memory in rodents, whereas inhibiting these receptors could have the opposite effect, reducing working memory performance .
Metabolic Pathways
Bromocriptine is completely metabolized by the liver, primarily by hydrolysis of the amide bond to produce lysergic acid and a peptide fragment, both inactive and non-toxic . Bromocriptine is metabolized by cytochrome P450 3A4 and excreted primarily in the feces via biliary secretion .
Transport and Distribution
Bromocriptine is rapidly absorbed (about 28% to 37% of the total dose) after oral administration . It is metabolized by cytochrome P450 3A4 and excreted primarily in the feces via biliary secretion .
準備方法
合成経路と反応条件: ブロモクリプチンは、麦角アルカロイドから始まる一連の化学反応によって合成されます。メシル酸ブロモクリプチンの調製は、ブロモクリプチンをメタノールに溶解し、続いてメタンスルホン酸水溶液を加えることから始まります。 混合物を撹拌し、冷却し、ろ過し、エタノールで再結晶化してメシル酸ブロモクリプチンを得ます .
工業生産方法: ブロモクリプチンの工業生産は、同様のステップを踏みますが、より大規模に行われます。 このプロセスには、最終製品の収率と純度を高くするために、温度やpHなどの反応条件を精密に制御することが含まれます .
化学反応の分析
反応の種類: ブロモクリプチンは、以下を含むさまざまな化学反応を起こします。
酸化: ブロモクリプチンは、特定の条件下で酸化されて、さまざまな代謝産物を生成する可能性があります。
還元: 還元反応は、分子中の臭素原子を修飾することができます。
一般的な試薬と条件:
酸化: 一般的な酸化剤には、過マンガン酸カリウムと過酸化水素が含まれます。
還元: 水素化アルミニウムリチウムなどの還元剤を使用することができます。
主な生成物: これらの反応から生成される主な生成物は、使用される特定の条件と試薬によって異なります。 たとえば、酸化により、さまざまなヒドロキシル化代謝産物が生成される可能性があります .
4. 科学研究への応用
ブロモクリプチンは、幅広い科学研究への応用があります。
化学: 新しい分析方法の開発における分析化学の基準化合物として使用されます.
生物学: ブロモクリプチンは、ドーパミン受容体の機能とシグナル伝達経路を研究するために使用されます。
類似化合物との比較
Cabergoline: Another dopamine agonist used to treat hyperprolactinemia and Parkinson’s disease.
Pergolide: Used in the treatment of Parkinson’s disease.
Quinagolide: A non-ergoline dopamine agonist used to treat hyperprolactinemia.
Comparison: Bromocriptine is unique in its quick-release formulation, which allows for rapid onset of action. It is also used as an adjunct therapy for type 2 diabetes, a feature not commonly seen with other dopamine agonists. Cabergoline, on the other hand, has a longer half-life and is often preferred for its less frequent dosing schedule .
特性
IUPAC Name |
(6aR,9R)-5-bromo-N-[(1S,2S,4R,7S)-2-hydroxy-7-(2-methylpropyl)-5,8-dioxo-4-propan-2-yl-3-oxa-6,9-diazatricyclo[7.3.0.02,6]dodecan-4-yl]-7-methyl-6,6a,8,9-tetrahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C32H40BrN5O5/c1-16(2)12-24-29(40)37-11-7-10-25(37)32(42)38(24)30(41)31(43-32,17(3)4)35-28(39)18-13-20-19-8-6-9-22-26(19)21(27(33)34-22)14-23(20)36(5)15-18/h6,8-9,13,16-18,23-25,34,42H,7,10-12,14-15H2,1-5H3,(H,35,39)/t18-,23-,24+,25+,31-,32+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OZVBMTJYIDMWIL-AYFBDAFISA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)CC1C(=O)N2CCCC2C3(N1C(=O)C(O3)(C(C)C)NC(=O)C4CN(C5CC6=C(NC7=CC=CC(=C67)C5=C4)Br)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)C[C@H]1C(=O)N2CCC[C@H]2[C@]3(N1C(=O)[C@](O3)(C(C)C)NC(=O)[C@H]4CN([C@@H]5CC6=C(NC7=CC=CC(=C67)C5=C4)Br)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C32H40BrN5O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
22260-51-1 (mesylate (salt)) | |
| Record name | Bromocriptine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0025614033 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID1022687 | |
| Record name | Bromocriptine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1022687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
654.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Bromocriptine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015331 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
8.58e-02 g/L | |
| Record name | Bromocriptine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015331 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The dopamine D2 receptor is a 7-transmembrane G-protein coupled receptor associated with Gi proteins. In lactotrophs, stimulation of dopamine D2 receptor causes inhibition of adenylyl cyclase, which decreases intracellular cAMP concentrations and blocks IP3-dependent release of Ca2+ from intracellular stores. Decreases in intracellular calcium levels may also be brought about via inhibition of calcium influx through voltage-gated calcium channels, rather than via inhibition of adenylyl cyclase. Additionally, receptor activation blocks phosphorylation of p42/p44 MAPK and decreases MAPK/ERK kinase phosphorylation. Inhibition of MAPK appears to be mediated by c-Raf and B-Raf-dependent inhibition of MAPK/ERK kinase. Dopamine-stimulated growth hormone release from the pituitary gland is mediated by a decrease in intracellular calcium influx through voltage-gated calcium channels rather than via adenylyl cyclase inhibition. Stimulation of dopamine D2 receptors in the nigrostriatal pathway leads to improvements in coordinated muscle activity in those with movement disorders. | |
| Record name | Bromocriptine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01200 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
25614-03-3 | |
| Record name | (+)-Bromocriptine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=25614-03-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Bromocriptine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0025614033 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bromocriptine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01200 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bromocriptine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1022687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Bromocriptine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.042.829 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | BROMOCRIPTINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3A64E3G5ZO | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Bromocriptine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015331 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
215-218 | |
| Record name | Bromocriptine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01200 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![(2S)-1-[(2S)-2-[[5-(2,3-dihydro-1-benzofuran-2-yl)-1-ethoxy-1-oxopentan-2-yl]amino]propanoyl]pyrrolidine-2-carboxylic acid](/img/structure/B1667802.png)
![6,6-Dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,3,5-triazine-2,4-diamine hydrochloride](/img/structure/B1667809.png)