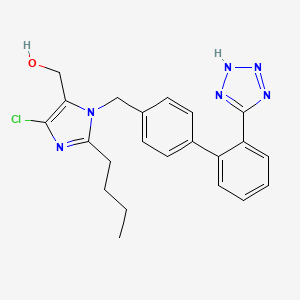
ロサルタン
概要
説明
Losartan is a medication primarily used to treat high blood pressure (hypertension) and to protect the kidneys from damage due to diabetesLosartan works by blocking the action of angiotensin II, a substance in the body that causes blood vessels to tighten, thereby relaxing blood vessels and lowering blood pressure .
作用機序
Target of Action
Losartan primarily targets the angiotensin II type 1 (AT1) receptor . The AT1 receptor is found in many tissues, including vascular smooth muscle and the adrenal gland . It plays a crucial role in regulating blood pressure and fluid balance .
Mode of Action
Losartan works by reversibly and competitively blocking the binding of angiotensin II to the AT1 receptor . This prevents the vasoconstricting and aldosterone-secreting effects of angiotensin II . Losartan and its active metabolite bind the AT1 receptor with 1000 times more affinity than they bind to the AT2 receptor .
Biochemical Pathways
The primary biochemical pathway affected by losartan is the renin-angiotensin system (RAS) . By blocking the AT1 receptor, losartan inhibits the effects of angiotensin II, leading to vasodilation and a reduction in total peripheral resistance . This results in a decrease in blood pressure .
Pharmacokinetics
Losartan’s pharmacokinetic properties include its absorption, distribution, metabolism, and excretion (ADME). It has a bioavailability of 25–35% . It is primarily bound to albumin in the blood and is metabolized in the liver by the CYP2C9 and CYP3A4 enzymes . The elimination half-life of losartan is 1.5–2 hours, and it is excreted via the kidneys (13–25%) and bile duct (50–60%) .
Result of Action
The molecular and cellular effects of losartan’s action include a decrease in total peripheral resistance (afterload) and cardiac venous return (preload) . This leads to a reduction in blood pressure . Losartan also reprograms the tumor microenvironment, leading to increased vascular perfusion, and enhances immune effector cell intratumoral infiltration and function .
Action Environment
Environmental factors can influence the action, efficacy, and stability of losartan. For instance, losartan is frequently detected in wastewater effluents, posing considerable risks to both aquatic ecosystems and human health . Furthermore, losartan doesn’t cause sensitivity to the sun
科学的研究の応用
Losartan has a wide range of scientific research applications:
Chemistry: Used as a model compound in the study of angiotensin II receptor blockers.
Biology: Investigated for its effects on cellular processes and signaling pathways.
Industry: Utilized in the development of controlled-release drug delivery systems.
生化学分析
Biochemical Properties
Losartan is a selective, competitive angiotensin II receptor type 1 (AT1) antagonist . It reduces the end organ responses to angiotensin II . Losartan administration results in a decrease in total peripheral resistance (afterload) and cardiac venous return (preload) .
Cellular Effects
Losartan is used to treat high blood pressure (hypertension) and is considered protective of the kidneys . It works by blocking angiotensin II, which tightens the blood vessels, allowing the blood to flow more smoothly and the heart to pump more efficiently .
Molecular Mechanism
Losartan works by blocking the action of angiotensin II . It is a selective and competitive, nonpeptide angiotensin II receptor antagonist . Losartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II . It interacts reversibly at the AT1 and AT2 receptors of many tissues and has slow dissociation kinetics .
Temporal Effects in Laboratory Settings
Losartan has been shown to have a significant effect over time in laboratory settings . In a study, it was observed that a significant improvement in SpO2/FiO2 ratio was observed from day 1 to 7 .
Dosage Effects in Animal Models
In animal models, the effects of Losartan vary with different dosages . For instance, in a study, it was found that the combination therapy of Losartan and another drug significantly decreased systolic blood pressure and mean arterial pressure without significant reflex tachycardia .
Metabolic Pathways
The major metabolic pathway for Losartan is by the cytochrome P450 (CYP) 3A4, 2C9, and 2C10 isoenzymes . Approximately 14% of a Losartan dose is converted to the pharmacologically active E 3174 metabolite .
Transport and Distribution
Losartan is rapidly and almost completely absorbed after oral administration, reaching maximum concentrations 1–2 hours post-administration . After oral administration, approximately 14% of a Losartan dose is converted to the pharmacologically active E 3174 metabolite .
準備方法
Synthetic Routes and Reaction Conditions: The synthesis of Losartan involves several key steps. One efficient and green synthetic route includes the preparation of two key intermediates: 2-butyl-4-chloro-3H-imidazole-5-carbaldehyde and 2-cyano-4’-methyl biphenyl. The former is synthesized from valeronitrile and acetyl chloride through a three-step process, while the latter is obtained by coupling o-chlorobenzonitrile with p-methylphenylmagnesium chloride in tetrahydrofuran in the presence of manganese chloride and chlorotrimethylsilane .
Industrial Production Methods: In industrial settings, the synthesis of Losartan is optimized for higher yields and efficiency. The process involves the use of sodium azide and triethylamine hydrochloride salt to construct the tetrazole ring from the cyano group . This method is preferred due to its practicality and efficiency in large-scale production.
化学反応の分析
Types of Reactions: Losartan undergoes various chemical reactions, including:
Oxidation: Losartan can be oxidized to form its active metabolite, EXP3174.
Reduction: Reduction reactions are less common but can occur under specific conditions.
Substitution: Losartan can undergo substitution reactions, particularly in the presence of strong nucleophiles.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include potassium permanganate and hydrogen peroxide.
Substitution: Reagents such as sodium azide and triethylamine hydrochloride are used to introduce the tetrazole ring.
Major Products:
EXP3174: The primary active metabolite formed through oxidation.
Tetrazole Derivatives: Formed through substitution reactions involving the cyano group.
類似化合物との比較
Losartan is compared with other angiotensin II receptor blockers such as:
Telmisartan: Known for its longer half-life and higher binding affinity to the AT1 receptor.
Valsartan: Similar in efficacy but with a different pharmacokinetic profile.
Irbesartan, Candesartan, and Olmesartan: Each has unique properties in terms of efficacy, safety, and duration of action.
Uniqueness of Losartan:
Metabolite Activity: The active metabolite, EXP3174, enhances its therapeutic effects.
Safety Profile: Losartan is well-tolerated with fewer side effects compared to some other angiotensin II receptor blockers.
Losartan remains a vital medication in the management of hypertension and related conditions, with ongoing research exploring its full potential in various scientific fields.
特性
IUPAC Name |
[2-butyl-5-chloro-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PSIFNNKUMBGKDQ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCCC1=NC(=C(N1CC2=CC=C(C=C2)C3=CC=CC=C3C4=NNN=N4)CO)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H23ClN6O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
124750-99-8 (mono-potassium salt) | |
| Record name | Losartan [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0114798264 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID7023227 | |
| Record name | Losartan | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023227 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
422.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Losartan | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014816 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
<1mg/mL, 4.70e-03 g/L | |
| Record name | Losartan | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00678 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Losartan | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014816 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Losartan reversibly and competitively prevents angiotensin II binding to the AT1 receptor in tissues like vascular smooth muscle and the adrenal gland. Losartan and its active metabolite bind the AT1 receptor with 1000 times more affinity than they bind to the AT2 receptor. The active metabolite of losartan is 10-40 times more potent by weight than unmetabolized losartan as an inhibitor of AT1 and is a non-competitive inhibitor. Losartan's prevention of angiotensin II binding causes vascular smooth muscle relaxation, lowering blood pressure. Angiotensin II would otherwise bind to the AT1 receptor and induce vasoconstriction, raising blood pressure., Angiotensin II (formed from angiotensin I in a reaction catalyzed by angiotensin converting enzyme (ACE, kininase II)), is a potent vasoconstrictor, the primary vasoactive hormone of the renin-angiotensin system and an important component in the pathophysiology of hypertension. It also stimulates aldosterone secretion by the adrenal cortex. Losartan and its principal active metabolite block the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor found in many tissues, (e.g., vascular smooth muscle, adrenal gland). There is also an AT2 receptor found in many tissues but it is not known to be associated with cardiovascular homeostasis. Both losartan and its principal active metabolite do not exhibit any partial agonist activity at the AT1 receptor and have much greater affinity (about 1000-fold) for the AT1 receptor than for the AT2 receptor. In vitro binding studies indicate that losartan is a reversible, competitive inhibitor of the AT1 receptor. The active metabolite is 10 to 40 times more potent by weight than losartan and appears to be a reversible, non-competitive inhibitor of the AT1 receptor. Neither losartan nor its active metabolite inhibits ACE (kininase II, the enzyme that converts angiotensin I to angiotensin II and degrades bradykinin); nor do they bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation., We investigated the effects of angiotensin II (Ang II) type 1 receptor blockade with losartan on the renin-angiotensin-aldosterone system in hypertensive patients (supine diastolic blood pressure, 95 to 110 mm Hg). Qualifying patients (n = 51) were allocated to placebo, 25 or 100 mg losartan, or 20 mg enalapril. Blood pressure, plasma drug concentrations, and renin-angiotensin-aldosterone system mediators were measured on 4 inpatient days: end of placebo run-in, after first dose, and 2 and 6 weeks of treatment. Plasma drug concentrations were similar after the first and last doses of losartan. At 6 weeks, 100 mg losartan and 20 mg enalapril showed comparable antihypertensive activity. Four hours after dosing, compared with the run-in day, 100 mg losartan increased plasma renin activity 1.7-fold and Ang II 2.5-fold, whereas enalapril increased plasma renin activity 2.8-fold and decreased Ang II 77%. Both drugs decreased plasma aldosterone concentration. For losartan, plasma renin activity and Ang II increases were greater at 2 than at 6 weeks. Effects of losartan were dose related. After the last dose of losartan, plasma renin activity and Ang II changes were similar to placebo changes by 36 hours. These results indicate that long-term blockade of the feedback Ang II receptor in hypertensive patients produces modest increases of plasma renin activity and Ang II that do not appear to affect the antihypertensive response to the antagonist. /Salt not specified/, IL-1beta is a potent proinflammatory, pro-fibrogenetic and pro-athrosclerosis cytokine which has been shown to play an important role in an expanding number of noninfectious, chronic inflammatory conditions including cardiovascular disease, renal fibrosis, rheumatoid arthritis and even type 2 diabetes. Losartan is an angiotensin II receptor antagonist widely used for the treatment of hypertension, diabetic nephropathy and congestive heart failure. In this study, we attempted to clarify whether losartan has an inhibitory effect on IL-1beta. To further elucidate the molecular mechanism underlying the anti-IL-1beta property of losartan, we studied the LPS+ATP-induced activation of NALP3 inflammasome which controls the muturation and secretion of IL-1beta. LPS and ATP were used to stimulate the release of IL-1beta from thioglycollate-elicited macrophages from BALB/c mice. The production of IL-1beta was evaluated by ELISA assay and NALP3, caspase-1, IL-beta mRNA levels were determined by reverse transcription-polymerase chain reaction. In cultured thioglycollate-elicited macrophages, we observed that LPS + ATP greatly enhanced IL-1 beta secretion (6938.00 +/- 83.45; P < 0.05) and the mRNA levels of NALP3, caspase-1 which are two main components of NALP3 inflammasome (60.88 +/- 8.28; 1.31 +/- 0.04, P < 0.05 for both). The macrophages co-cultured with losartan showed low production of IL-1beta (3907.50 +/- 143.61; P < 0.05) and low production of NALP3, caspase-1mRNA (29.82 +/- 6.92; 1.12 +/- 0.05, P < 0.05 for both). Losartan did not reduce IL-1beta mRNA(P > 0.05). Our results show that the NALP3 inflammasome is up-regulated and activated in the mouse macrophage in response to LPS + ATP stimulation. Losartan is able to suppress the LPS + ATP-induced production of IL-1beta protein. In addition, this effectmay be partially mediated by suppressing NALP3 inflammasome activation., The present study aimed to investigate the molecular pharmacodynamic mechanisms of losartan used in the treatment of hypertension. A total of 12 spontaneously hypertensive rats (SHR) were divided randomly into an SHR group treated with saline and LOS group treated with losartan. Six Wistar-kyoto rats (WKY) were enrolled as the WKY group with saline in the study. The LOS group received 30 mg/kg/day losartan by intragastric injection, while the SHR and WKY were fed the same volume of saline. The dosage was modulated according to the weekly weight. Changes in blood pressure were measured by the indirect tail cuff method. Angiotensin (Ang) II production in the plasma and renal tissue was measured by an immunoradiometric method. Na+/H+ exchanger (NHE)3 and serum and glucocorticoid-inducible kinase (SGK)1 were assessed by quantitative polymerase chain reaction (qPCR) and western blot analysis. When compared with the WKY group, the blood pressure of the SHR and LOS groups were higher prior to treatment with losartan. Following two weeks, blood pressure was reduced and the trend continued to decrease over the following six weeks. The plasma and renal tissue levels of Ang II in the SHR and LOS groups were significantly higher than those in the WKY group. NHE3 and SGK1 were increased at the mRNA and protein level in the SHR group, and losartan reduced the expression of both of them. The results suggested that in hypertensive rats, the circular and tissue renin angiotensin systems were activated, and the increased Ang II stimulated the expression of NHE3 and SGK1, which was reduced by losartan. Therefore, the effects of losartan in hypertension may be associated with the Ang II-SGK1-NHE3 of intra-renal tissue., For more Mechanism of Action (Complete) data for Losartan (7 total), please visit the HSDB record page. | |
| Record name | Losartan | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00678 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Losartan | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7043 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Light yellow solid | |
CAS No. |
114798-26-4 | |
| Record name | Losartan | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=114798-26-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Losartan [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0114798264 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Losartan | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00678 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | losartan | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758699 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Losartan | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023227 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.110.555 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LOSARTAN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/JMS50MPO89 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Losartan | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7043 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Losartan | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014816 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
178-184, 183.5-184.5 °C, 183.5 - 184.5 °C | |
| Record name | Losartan | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00678 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Losartan | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7043 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Losartan | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014816 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details




Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。