Procarbazine
概要
説明
準備方法
合成経路と反応条件: プロカルバジンの合成は、p-トルアルデヒドから始まるいくつかのステップを含みます。このプロセスには、トルイルイソプロピルアミンを得るために、ジブロモシアヌル酸とイソプロピルアミンを添加することが含まれます。この中間体は、次に有機試薬に溶解され、続いてN-ブロモスクシンイミドと開始剤を添加します。この混合物を還流まで加熱し、溶媒を除去します。アセトニトリルと加水分解促進剤を加え、この混合物を還流まで加熱して、ホルモキシルベンゾイルイソプロピルアミンを形成します。 最後に、ホルモキシルベンゾイルイソプロピルアミンをメチルヒドラジニウム硫酸塩とトリエチルアミンと反応させ、続いてシアノホウ素化ナトリウムを添加することにより、プロカルバジンが生成されます .
工業生産方法: プロカルバジンの工業生産は、同様の合成経路に従いますが、より高い収率と効率のために最適化されています。このプロセスでは、強力な酸化剤や強力な酸の使用を避け、環境に優しいものとなっています。 工業的方法の総回収率は約52.9%です .
化学反応の分析
反応の種類: プロカルバジンは、酸化、還元、置換など、さまざまな化学反応を起こします。注目すべき反応の1つは、アゾ誘導体を形成する自動酸化であり、続いてヒドラゾンに異性化します。 このヒドラゾンは、加水分解されてベンジルアルデヒド誘導体とメチルヒドラジンを生成します .
一般的な試薬と条件: プロカルバジンを含む反応で使用される一般的な試薬には、N-ブロモスクシンイミド、アセトニトリル、加水分解促進剤、メチルヒドラジニウム硫酸塩、シアノホウ素化ナトリウムなどがあります . 反応条件は、通常、還流まで加熱し、有機溶媒を使用することを含みます。
主な生成物: プロカルバジンの反応から生成される主な生成物には、ベンジルアルデヒド誘導体とメチルヒドラジンが含まれます .
科学研究アプリケーション
プロカルバジンは、化学、生物学、医学、産業などの分野で、幅広い科学研究アプリケーションを持っています。 医学では、ホジキンリンパ腫や脳腫瘍の治療のための併用化学療法レジメンの一部として使用されます . 化学では、プロカルバジンは、その独自のアルキル化特性と、薬物送達システムのためのさまざまなナノ構造との相互作用について研究されています . 生物学では、細胞プロセスやDNA合成に対するアルキル化剤の影響を研究するために使用されています . 産業用途には、他の医薬品化合物の合成における使用や、環境に優しい生産方法の開発における役割が含まれます .
科学的研究の応用
Procarbazine has a wide range of scientific research applications, particularly in the fields of chemistry, biology, medicine, and industry. In medicine, it is used as part of combination chemotherapy regimens for treating Hodgkin’s lymphoma and brain cancers . In chemistry, this compound is studied for its unique alkylating properties and its interactions with various nanostructures for drug delivery systems . In biology, it is used to study the effects of alkylating agents on cellular processes and DNA synthesis . Industrial applications include its use in the synthesis of other pharmaceutical compounds and its role in developing environmentally friendly production methods .
作用機序
プロカルバジンの正確な作用機序は、まだ完全に解明されていません。 メチオニンのメチル基を転移RNAに転移することを妨げることで、タンパク質、RNA、およびDNAの合成を阻害することが知られています . プロカルバジンは、アルキル化剤としても機能し、グアニンのO-6位をメチル化することで、DNAの切断とRNAおよびタンパク質合成の阻害につながります .
類似化合物との比較
類似化合物: プロカルバジンに類似した化合物には、ダカルバジン、ブレオマイシン、ニボルマブなどがあります . これらの化合物は、さまざまな癌の治療にも使用され、アルキル化剤または抗悪性腫瘍剤としての作用機序が類似しています。
独自性: プロカルバジンは、クロルメチン、ビンクリスチン、プレドニゾンなどの他の化学療法剤と組み合わせて使用することで、ホジキンリンパ腫の治療に用いることができるという点で独特です . また、薬物送達システムのためのナノ構造との相互作用においても異なり、癌細胞の標的化における有効性を高めています .
特性
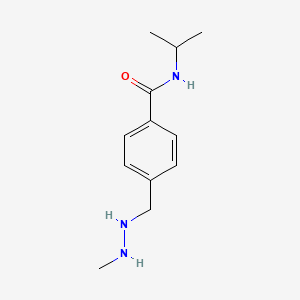
IUPAC Name |
4-[(2-methylhydrazinyl)methyl]-N-propan-2-ylbenzamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C12H19N3O/c1-9(2)15-12(16)11-6-4-10(5-7-11)8-14-13-3/h4-7,9,13-14H,8H2,1-3H3,(H,15,16) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CPTBDICYNRMXFX-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)NC(=O)C1=CC=C(C=C1)CNNC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C12H19N3O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
366-70-1 (mono-hydrochloride) | |
| Record name | Procarbazine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000671169 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID4021189 | |
| Record name | Procarbazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4021189 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
221.30 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Procarbazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015299 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
In water, 1,400 mg/L @ 25 °C /Estimated/, 2.28e-01 g/L | |
| Record name | Procarbazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01168 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PROCARBAZINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3250 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Procarbazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015299 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
8.4X10-7 mm Hg @ 25 °C /Estimated/ | |
| Record name | PROCARBAZINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3250 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
The precise mode of cytotoxic action of procarbazine has not been clearly defined. There is evidence that the drug may act by inhibition of protein, RNA and DNA synthesis. Studies have suggested that procarbazine may inhibit transmethylation of methyl groups of methionine into t-RNA. The absence of functional t-RNA could cause the cessation of protein synthesis and consequently DNA and RNA synthesis. In addition, procarbazine may directly damage DNA. Hydrogen peroxide, formed during the auto-oxidation of the drug, may attack protein sulfhydryl groups contained in residual protein which is tightly bound to DNA., Procarbazine is an alkylating agent. The exact mechanism of antineoplastic action is unknown but is thought to resemble that of the alkylating agents; procarbazine is cell cycle-specific for the S phase of cell division. Procarbazine is thought to inhibit DNA, RNA, and protein synthesis., O-6-Methylguanine was measured in blood leukocyte DNA of seven patients with Hodgkin's or non-Hodgkin's lymphoma during therapeutic exposure to procarbazine involving three daily p.o. doses (50 mg each) for 10 days (corresponding to 2.1 mg/kg/day for a 70-kg human). Adduct accumulation was observed in all seven cases, reaching levels up to 0.28 fmol/microgram of DNA (0.45 umol/mol of guanine). In one individual, maximal levels of adduct were reached after 7 days of exposure, followed by a steady decline, whereas in all other individuals continuous accumulation was observed throughout the exposure period. In four individuals for which data were available for day 11 (12 to 16 hr after the final intake of procarbazine), decreased amounts of O-6-methylguanine were observed relative to the last previous measurements. The accumulation of O-6-methylguanine was linearly correlated with the cumulative dose of procarbazine, with a slope of 0.011 fmol of O-6-methylguanine/microgram of DNA per mg/kg of body weight or 2.68x10-4 fmol of O-6-methylguanine DNA per mg/sq m. Two hr after the administration of single p.o. doses of l to 10 mg/kg of procarbazine to rats, O-6-methylguanine formation in leukocyte DNA was just under half that in liver DNA and showed a linear relationship with dose with a slope of 0.017 fmol/microgram of DNA per mg/kg of body weight or 5.67x10-4 fmol of O-6-methylguanine/microgram of DNA per mg/sq m. A negative correlation between the rate of accumulation of O-6-methylguanine in different individuals and lymphocyte O-6-alkylguanine-DNA alkyltransferase was observed, demonstrating a probable protective effect of O-6-alkylguanine-DNA alkyltransferase against the accumulation of O-6-methylguanine during exposure to methylating agents. This observation supports the suggestion of a possible role of procarbazine-induced O-6-methylguanine in the pathogenesis of acute nonlymphocytic leukemia appearing after treatment with chemotherapeutic protocols which include procarbazine, based on the finding of low lymphocyte O-6-alkylguanine-DNA alkyltransferase levels in patients with such therapy-related neoplastic disease. Lymphocyte O-6-alkylguanine-DNA alkyltransferase levels were mainly in the range of 5 to 10 fmol/micrograms of DNA and showed no consistent variation during procarbazine exposure., Procarbazine causes weak inhibition of monoamine oxidase (MAO). MAO inhibitors prevent the inactivation of tyramine by hepatic and gastrointestinal monoamine oxidase. Tyramine in the bloodstream releases norepinephrine from the sympathetic nerve terminals and produces a sudden increase in blood pressure. | |
| Record name | Procarbazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01168 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PROCARBAZINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3250 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
671-16-9, 366-70-1 | |
| Record name | Procarbazine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=671-16-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Procarbazine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000671169 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Procarbazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01168 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Procarbazine hydrochloride | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759626 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Procarbazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4021189 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Procarbazine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.010.531 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PROCARBAZINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/35S93Y190K | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PROCARBAZINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3250 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Procarbazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015299 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
223 °C | |
| Record name | Procarbazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01168 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Procarbazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015299 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。